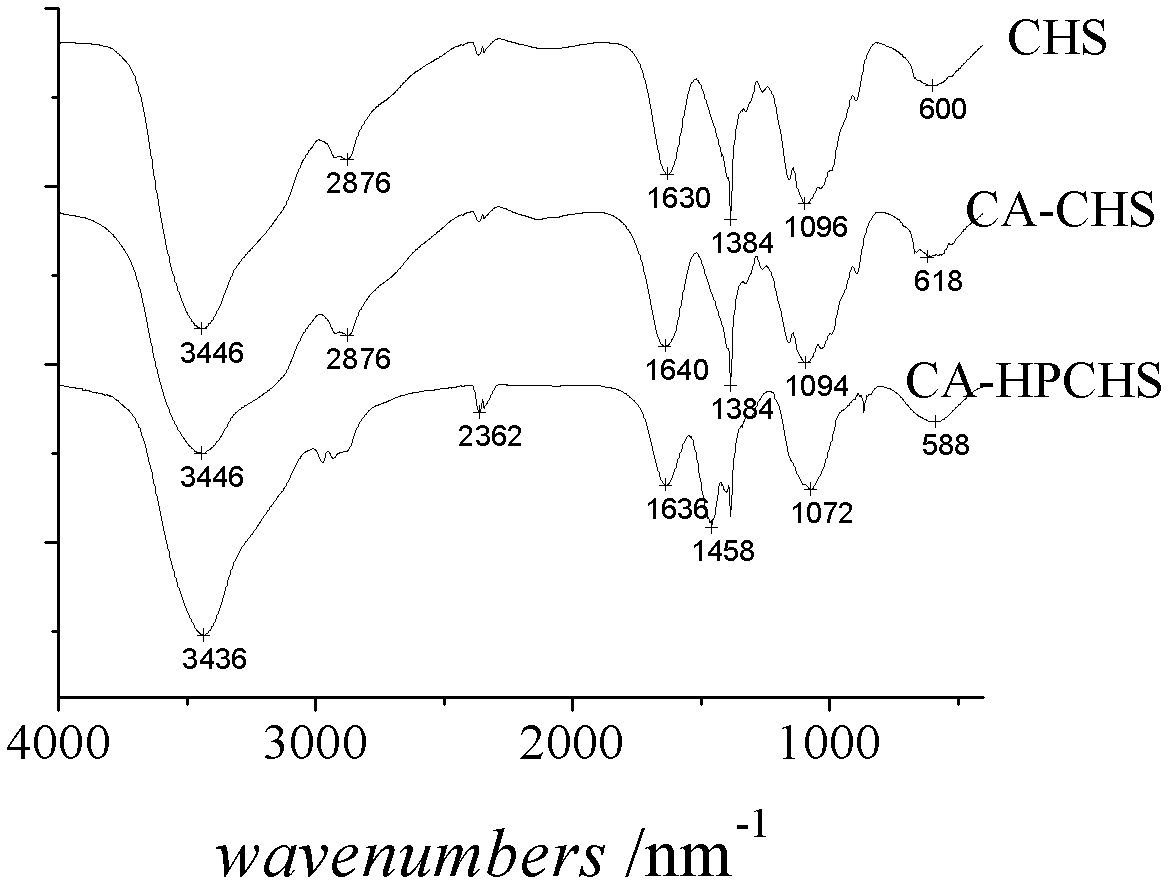
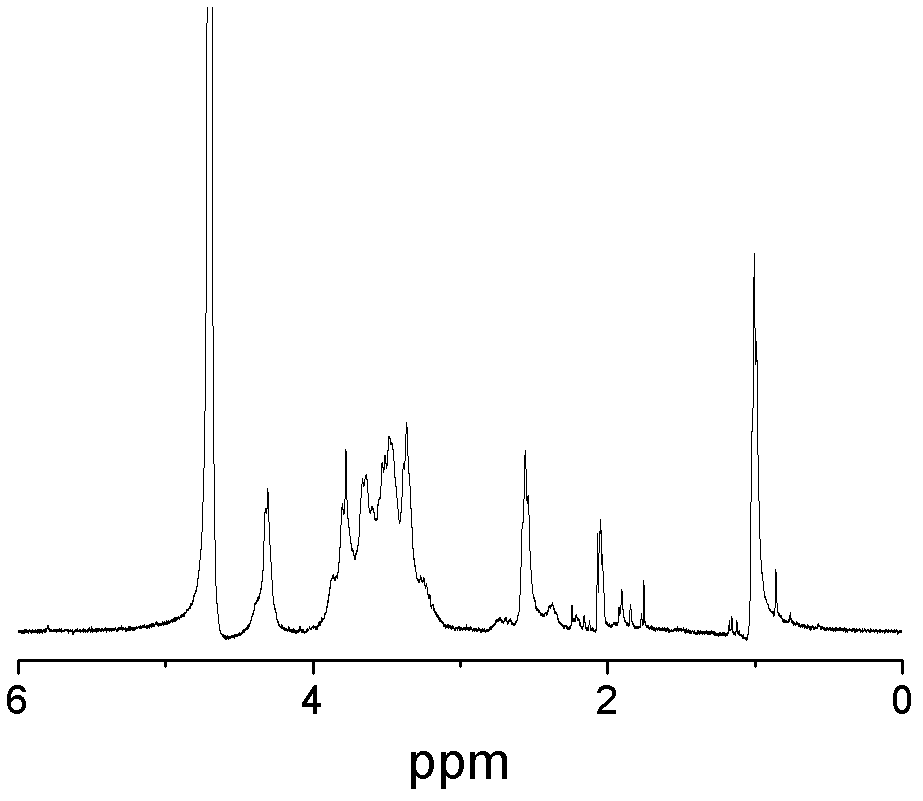
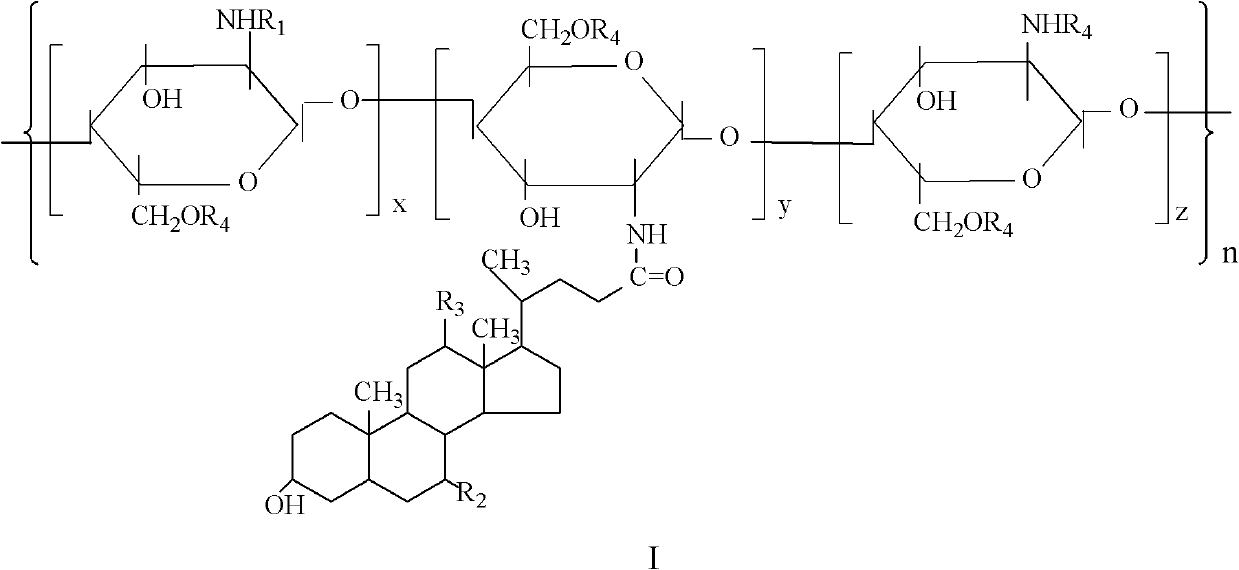
A kind of amphiphilic chitosan-bile acid derivative and preparation method thereof
A bile acid derivative, chitosan technology, applied in the field of biomedicine, can solve the problems of poor water solubility, no solubilization and emulsifying ability of chitosan, and achieve good surface activity, good biocompatibility, non-toxicity, good The effect of biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The amphiphilic chitosan-bile acid derivative general formula of the present embodiment is as described in the summary of the invention, wherein, R 2 , R 3 Both are -OH, R 4 for -CH 2 CHOHCH 3 .
[0030] The preparation method is as follows:
[0031] 1. Dissolve 1.0g of chitosan with 100ml of 1% acetic acid, then add 100ml of methanol solution containing 0.76g of cholic acid, stir well, add 0.072g of EDC and 0.086g of NHS and react at 25°C for 24h. The product was neutralized by adding 10ml of ammonia water, added with 200ml of methanol for precipitation, filtered, the filter cake was fully soaked in acetone for dehydration, then washed with acetone and then vacuum-dried to obtain cholic acid-chitosan.
[0032] 2. Disperse the cholic acid-chitosan prepared in step 1 in 10ml alkaline isopropanol solution for alkalization for 10h. The alkaline isopropanol solution is 35wt% NaOH and isopropanol in a volume ratio of 1:9 the mix of. Add 4.4ml of propylene oxide to the...
Embodiment 2
[0049] The amphiphilic chitosan-bile acid derivative general formula of the present embodiment is as described in the summary of the invention, wherein, R 2 for -H,R 3 for -OH, R 4 for -CH 2 CHOHCH 3 .
[0050] The preparation method is as follows:
[0051] 1. Dissolve 1.0g of chitosan with 100ml of 1% acetic acid, then add 100ml of methanol solution containing 0.73g of deoxycholic acid, stir well, add 0.072g of EDC and 0.086g of NHS and react at 25°C for 24h. The product was neutralized by adding 10 ml of ammonia water, added 200 ml of methanol to precipitate, filtered, the filter cake was fully soaked in acetone for dehydration, then washed with acetone and dried in vacuum to obtain deoxycholic acid-chitosan.
[0052] 2. Disperse the deoxycholic acid-chitosan prepared in step 1 in 10ml alkaline isopropanol solution for alkalization for 10h. The alkaline isopropanol solution is 35wt% NaOH and isopropanol in a volume ratio of 1:9 the mix of. Add 4.4ml of propylene oxide...
Embodiment 3
[0054] The amphiphilic chitosan-bile acid derivative general formula of the present embodiment is as described in the summary of the invention, wherein, R 2 , R 3 Both are -H, R 4 for -CH 2 CHOHCH 3 .
[0055] The preparation method is as follows:
[0056] 1. Dissolve 1.0g of chitosan with 100ml of 1% acetic acid, then add 100ml of methanol solution containing 0.70g of lithocholic acid, stir well, add 0.072g of EDC and 0.086g of NHS and react at 25°C for 24h. Add 10ml of ammonia water to the product, add 200ml of methanol to precipitate, filter, fully soak the filter cake in acetone for dehydration, then wash with acetone and vacuum dry to obtain lithocholic acid-chitosan.
[0057] 2, disperse the lithocholic acid-chitosan that step 1 makes in 10ml alkaline isopropanol solution and basify for 10h, the alkaline isopropanol solution is 35wt% NaOH and isopropanol in a volume ratio of 1:9 the mix of. Add 4.4ml of propylene oxide to the solution, reflux in a water bath at 45...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com