Rapid, simple, convenient and sensitive detection method for related impurities of glucosamine salt medicines
A technology for glucosamine and related impurities, which is applied in the field of fast, simple and sensitive detection of impurities related to glucosamine salt drugs, can solve the problems of difficult to detect impurities, low sensitivity of thin-layer detection, poor specificity, etc. The effect of strong exclusivity and security
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
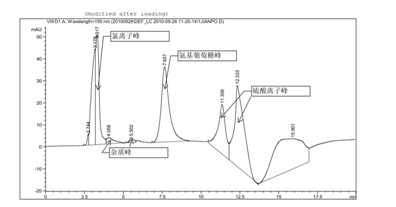
[0034] Glucosamine sulfate potassium chloride (provided by Jiangsu Shuanglin Marine Bio-Pharmaceutical Co., Ltd., batch number: 20100425) detection of impurities related to alkali destruction
[0035] Chromatographic conditions and system adaptability test use amino column as detection column; use phosphate buffer (take 0.7gKH 2 PO 4 , add water to dilute to 1000ml, adjust pH to 7.0 with concentrated ammonia solution)-acetonitrile (volume ratio is 35: 65) is mobile phase; Determination wavelength is 195nm. Flow rate: 1.0ml / min; column temperature: 35°C. The number of theoretical plates is not less than 800 based on glucosamine hydrochloride.
[0036]Determination method Take the sample solution neutralized by hydrochloric acid after being destroyed by 1N sodium hydroxide alkali for 30 minutes (glucosamine is unstable under alkaline conditions), dilute it with water to a solution containing about 10 mg per 1 ml, and filter it with a 0.45 μm filter membrane , as the test solu...
Embodiment 2
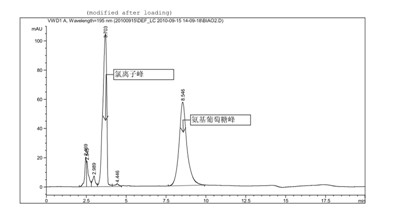
[0039] Glucosamine hydrochloride (provided by Jiangsu Shuanglin Marine Bio-Pharmaceutical Co., Ltd., batch number: 20100302, the sample is double-packed, lined with polyethylene plastic bags, sealed with aluminum foil, stored in a cool, dry place) related impurities detection solid Samples were stored under high humidity conditions.
[0040] Chromatographic conditions and system adaptability test use amino column as detection column; use phosphate buffer (take 0.7gKH 2 PO 4 , add water to dilute to 1000ml, adjust pH to 7.5 with concentrated ammonia solution)-acetonitrile (volume ratio 35:65) is mobile phase; Determination wavelength is 195nm. Flow rate: 1.0ml / min; column temperature: 35°C. The number of theoretical plates is not less than 800 based on glucosamine hydrochloride.
[0041] Determination method Take an appropriate amount of sample, weigh it accurately, dissolve it in water and make a solution containing about 10 mg per 1 ml, filter it with a 0.45 μm filter memb...
Embodiment 3
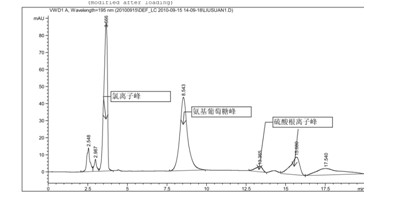
[0044] Glucosamine sulfate potassium chloride (provided by Jiangsu Shuanglin Marine Biological Pharmaceutical Co., Ltd., batch number: 20101008, the sample is double-packed, lined with polyethylene plastic bags, sealed with aluminum foil on the outer cover, and stored in a cool, dry place) About impurity detection
[0045] Chromatographic conditions and system adaptability test use amino column as detection column; use phosphate buffer (take 0.7gKH 2 PO 4 , add water to dilute to 1000ml, adjust pH to 7.5 with concentrated ammonia solution)-acetonitrile (volume ratio 35:65) is mobile phase; Determination wavelength is 195nm. Flow rate: 1.0ml / min; column temperature: 35°C. The number of theoretical plates is not less than 800 based on glucosamine hydrochloride.
[0046] Determination method Take an appropriate amount of sample, weigh it accurately, dissolve it in water and make a solution containing about 10 mg per 1 ml, filter it with a 0.45 μm filter membrane, and use it as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com