Polyethyleneimine derivative and application thereof as gene delivery carrier
A technology of polyethylenimine and its derivatives, which is applied in the direction of introducing foreign genetic material using carriers and recombinant DNA technology. The method is simple and easy to perform, and the effect of high transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of Embodiment 1 Cyclic Phosphate Ester Monomer
[0054] The cyclic phosphate monomer is prepared by reacting 2-chloro-2-oxo-1,3,2-dioxaphospholane with absolute ethanol. The specific steps are as follows: After mixing 4.6g of absolute ethanol with 150mL of THF, add 10.1g of triethylamine, cool at 0°C for 20min, then add 2-chloro-2-oxo-1,3,2-dioxo Phospholane (14.8 g) in THF was reacted overnight at this temperature. After filtering, the triethylamine hydrochloride precipitate was removed, and the filtrate was concentrated and then distilled under reduced pressure to obtain the final product.
Embodiment 2
[0055] Synthesis and characterization of embodiment 2 polyethyleneimine derivatives
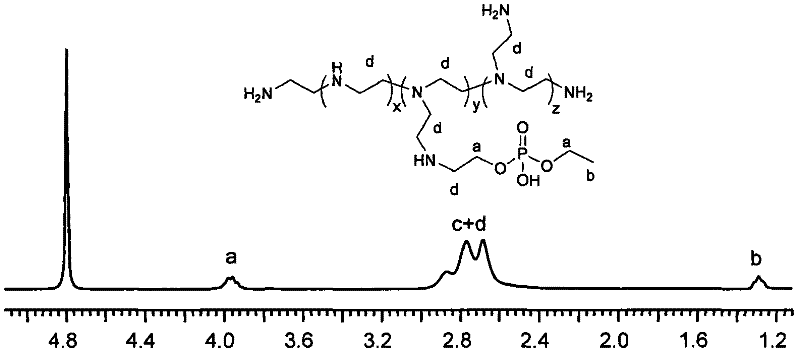
[0056] The preparation of polyethyleneimine derivatives was carried out under anhydrous environment. Concrete steps are as follows: 2g polyethyleneimine (M w =25000g / mol) was dissolved in 4mL dry DMSO, stirred at 50°C to fully dissolve, then 0.48g cyclic phosphate monomer was added, and the reaction stopped after 2h at this temperature, and the viscosity of the system increased significantly. After adding a small amount of methanol for dilution, the system was precipitated into excess ether, and after stirring for 3 hours, the supernatant was discarded to remove the unreacted cyclic phosphate monomer, and the precipitate was collected and dried under reduced pressure. This polymer was redissolved in deionized water and then dialyzed for two days (MWCO = 2000 Da). After the solution was lyophilized, it was stored at -80°C, polyethyleneimine derivative P1.
[0057] It was determined that the...
Embodiment 3
[0059] Embodiment 3 polyethyleneimine derivatives
[0060] The preparation of polyethyleneimine derivatives was carried out under anhydrous environment. Concrete steps are as follows: 2.0g polyethyleneimine (M w =1800g / mol) was dissolved in 4mL of dry DMSO, stirred at 40°C to fully dissolve, then 1.0g of cyclic phosphate monomer was added, and the reaction stopped after 3h at this temperature, and the viscosity of the system increased significantly. After adding a small amount of methanol for dilution, the system was precipitated into excess ether, and after stirring for 2 hours, the supernatant was discarded to remove the unreacted cyclic phosphate monomer, and the precipitate was collected and dried under reduced pressure. This polymer was redissolved in deionized water and dialyzed for two days (MWCO = 1000 Da). After the solution was lyophilized, it was stored at -80°C, polyethyleneimine derivative P2.
[0061] It has been determined that the grafting ratio of polyethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com