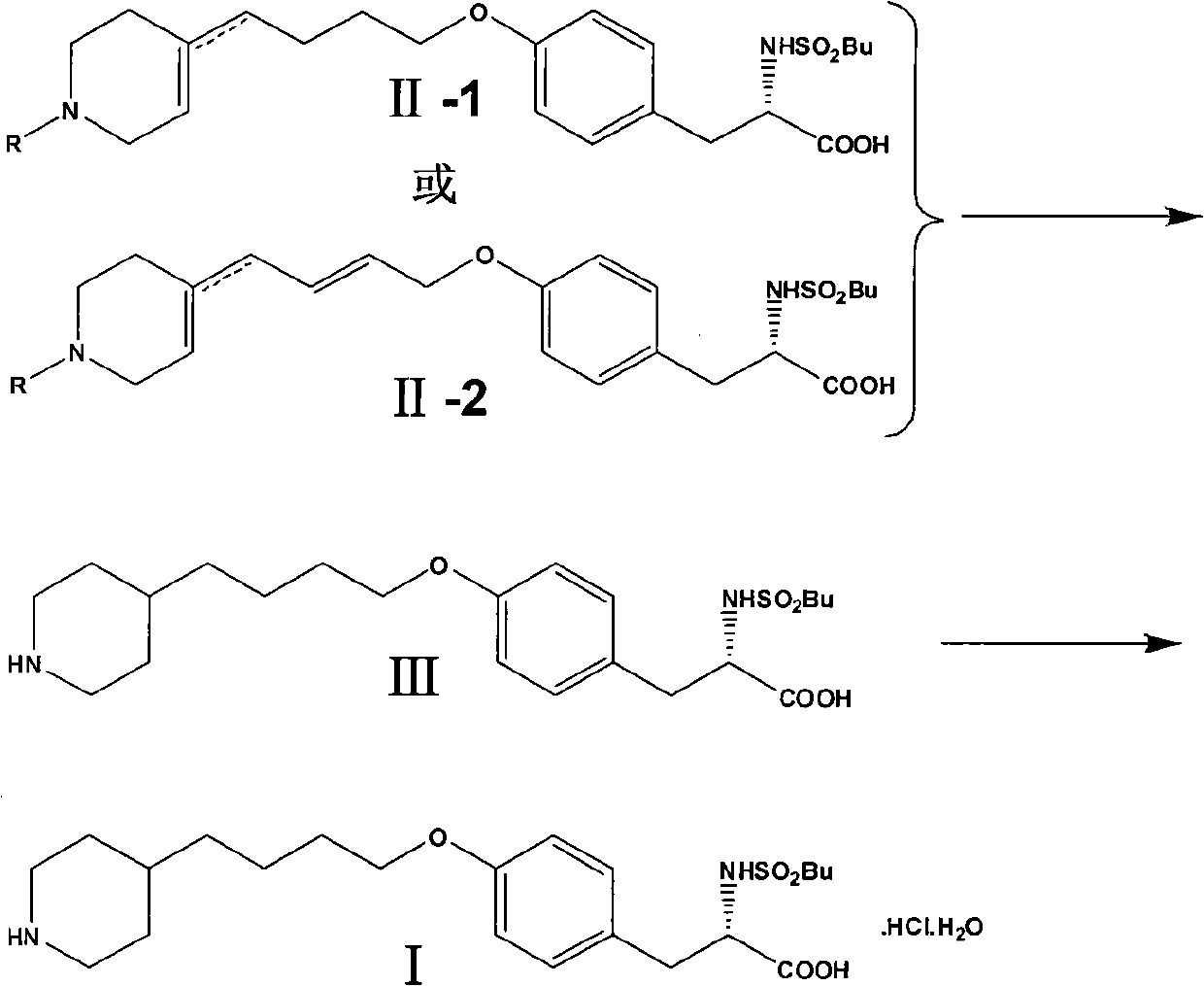
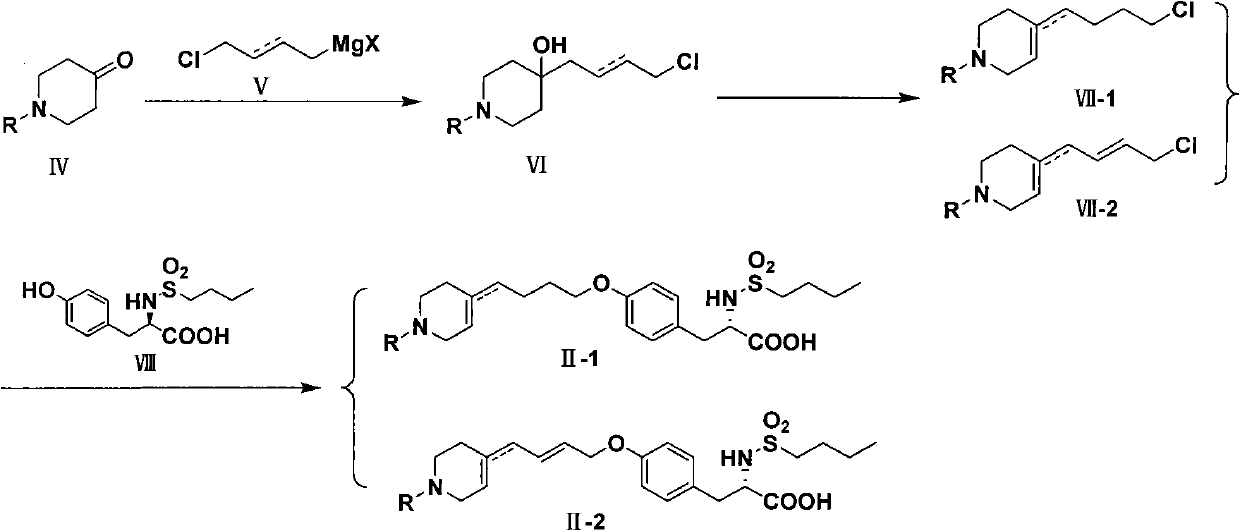
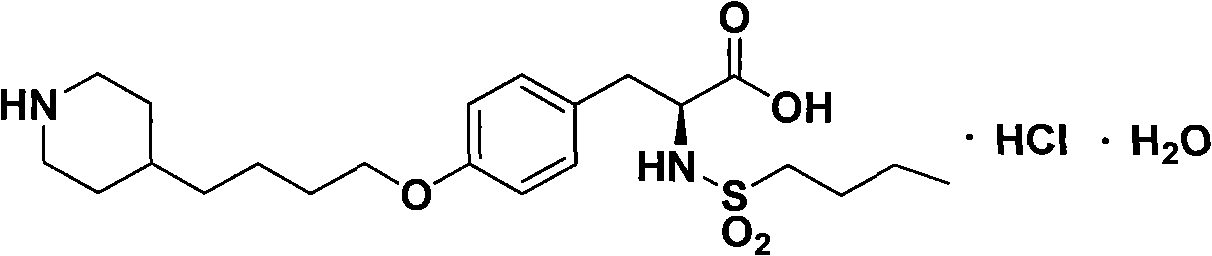
Method for preparing tirofiban hydrochloride
A technology for tirofiban and hydrochloric acid, which is applied in the field of preparation of tirofiban hydrochloride, can solve the problems of large solvent consumption, harsh reaction conditions, high risk, etc., and achieve easy treatment of three wastes, mild reaction conditions, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Synthesis of compound Ⅱ-1-1:
[0059] Take 1-benzyl-4-piperidone (50.0 g, 0.26 mol) and operate according to General Method 1 to obtain a mixture of isomers with and without double bonds II-1-1 with a yield of 64.8%. Through column separation, the pure products of the double bond product in the ring and the double bond product outside the ring can be obtained respectively (the eluent is CH 2 Cl 2 :CH 3 OH=50:1).
[0060] Intracyclic double bond product
[0061] 1 HNMR (400MHz, CDCl 3 )δ: 0.90(t, 3H), 1.31(m, 2H), 1.33(m, 2H), 1.75(m, 2H), 1.85(m, 2H), 1.96(m, 2H), 2.04-2.06(m , 4H), 2.39-2.55(m, 4H), 2.97(m, 2H), 2.98-3.16(m, 2H), 3.41(m, 2H), 3.62(s, 2H), 3.80(m, 1H), 4.01(m, 2H), 5.24(m, 1H), 6.74(d, 2H), 7.03(d, 2H)7.28-7.40(m, 5H)
[0062] MS (m / z): 529.2767 (M+H) + .
[0063] exocyclic double bond product
[0064] 1 HNMR (400MHz, CDCl 3 )δ: 0.88(t, 3H), 1.31(m, 2H), 1.58(m, 2H), 1.65(m, 2H), 1.75(m, 2H), 2.04-2.06(m, 4H), 2.39-2.55 (m, 4H), 2.97(m, 2H...
Embodiment 2
[0067] Synthesis of compound Ⅱ-1-2:
[0068] Benzyl 4-oxopiperidine-1-carboxylate (60.6 g, 0.26 mol) was operated according to General Method 1 to obtain a mixture of isomers with and without double bonds II-1-2 with a yield of 63.1%. Through column separation, the pure products of the product in the ring and the product outside the ring can be obtained respectively (the eluent is CH 2 Cl 2 :CH 3 OH=100:1).
[0069] Intracyclic double bond product
[0070] 1 HNMR (400MHz, CDCl 3 )δ: 0.90(t, 3H), 1.31(m, 2H), 1.33(m, 2H), 1.75(m, 2H), 1.85(m, 2H), 1.96(m, 2H), 2.21-2.29(m , 4H), 2.97(m, 2H), 2.98-3.16(m, 2H), 3.02-3.10(m, 4H), 3.41(m, 2H), 3.80(m, 1H), 4.01(m, 2H), 5.24(m, 1H), 5.34(s, 2H), 6.74(d, 2H), 7.03(d, 2H) 7.20(m, 5H)
[0071] MS (m / z): 573.2556 (M+H) + .
[0072] exocyclic double bond product
[0073] 1 HNMR (400MHz, CDCl 3 )δ: 0.88(t, 3H), 1.31(m, 2H), 1.58(m, 2H), 1.65(m, 2H), 1.75(m, 2H), 2.05(m, 2H), 2.97-3.12(m , 4H), 3.40(m, 2H), 3.44(s, 1H), 3.56(...
Embodiment 3
[0076] Synthesis of compound Ⅱ-1-3:
[0077] Take 1-allyl-4-piperidone (36.1 g, 0.26 mol) and operate according to General Method 1 to obtain a mixture of isomers with and without double bonds II-1-3 with a yield of 65.3%. Through column separation, the pure products of the product in the ring and the product outside the ring can be obtained respectively (the eluent is CH 2 Cl 2 :CH 3 OH=50:1).
[0078] Intracyclic double bond product
[0079] 1 HNMR (400MHz, CDCl 3 )δ: 0.90(t, 3H), 1.31(m, 2H), 1.33(m, 2H), 1.75(m, 2H), 1.85(m, 2H), 1.96(m, 2H), 2.11(m, 2H ), 2.44(m, 2H), 2.89(m, 2H), 3.06(m, 2H), 3.02-3.10(m, 2H), 3.41(m, 2H), 3.80(m, 1H), 4.01(m, 2H), 5.14-5.16(m, 2H), 5.85(m, 1H), 6.74(d, 2H), 7.03(d, 2H).
[0080] MS (m / z): 479.2498 (M+H) + .
[0081] exocyclic double bond product
[0082] 1 HNMR (400MHz, CDCl 3 )δ: 0.90(t, 3H), 1.31(m, 2H), 1.75(m, 2H), 1.85(m, 2H), 1.96(m, 2H), 2.06(m, 4H), 2.40(m, 4H ), 3.02(m, 2H), 3.02-3.10(m, 2H), 3.41(m, 2H), 3.80(m, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com