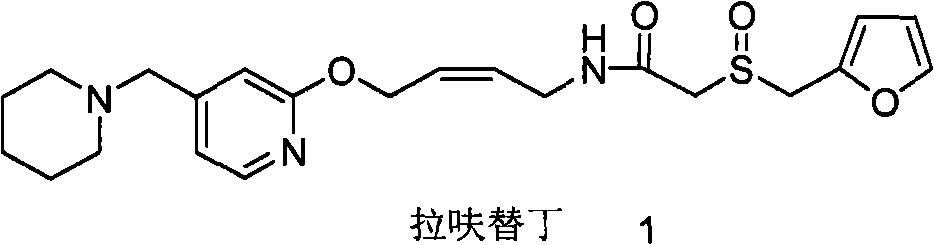
Reducing preparation method for lafutidine
A technology of lafutidine and a reducing agent, which is applied in the field of preparation of lafutidine, can solve the problems of reducing the total yield of synthetic lafutidine, high synthetic cost of lafutidine, etc., and simplify the post-processing process , avoid loss, improve the effect of synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0056] Experimental example: the effect of hydrazine on the production of dihydrolafutidine
[0057] In order to verify the effect of hydrazine on the production of dihydrolafutidine, the inventors designed and carried out the following experiments.
[0058] Dissolve 1 equivalent of the compound of formula 3 obtained by hydrazinolysis in a solvent, add 10 equivalents of hydrazine hydrate, heat and reflux for a certain period of time, evaporate the solvent to dryness, dissolve with ethyl acetate, wash with saturated sodium chloride solution, and dry over anhydrous magnesium sulfate. The compound of formula 3 obtained by evaporating ethyl acetate, further condenses with 2-furan methylsulfinyl acetate p-nitrophenol ester (compound of formula 4) to obtain lafutidine, check wherein dihydrolafutidine with liquid chromatography Ding impurity content.
[0059]
[0060] In the above test, the compound of formula 3 and the compound of formula 4 were condensed to obtain lafut...
Embodiment 1
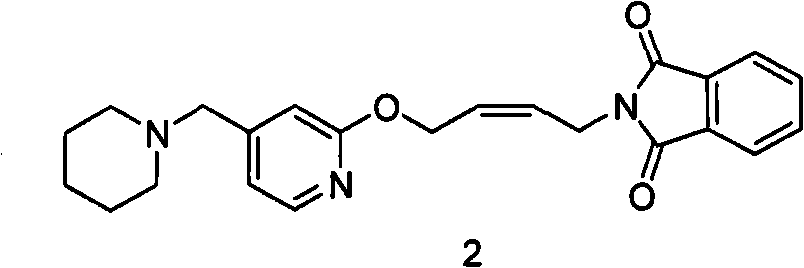
[0061] Example 1: N-(4-(4-piperidinylmethyl)pyridinyl-2-oxo)-2- Enyl) phthalimide (formula 2 compound) preparation
[0062] 1 kg of N-(4-(4-piperidinylmethyl)pyridyl-2-oxo)-2-enbutyl)phthalimide maleate was added into the 5L reactor, Add 2.7 liters of ethyl acetate and stir well. 1.3 liters of aqueous solution of 250 g of sodium hydroxide was added dropwise, and the temperature during the dropwise addition did not exceed 20°C. After dropping, continue to stir until the feed liquid is clear. The ethyl acetate phase was separated. Return the water phase to the reactor, add 0.5 liter of ethyl acetate, stir and extract, combine the ethyl acetate phase, add anhydrous magnesium sulfate, filter out the desiccant, distill under reduced pressure and recover ethyl acetate to obtain an oily substance.
Embodiment 2
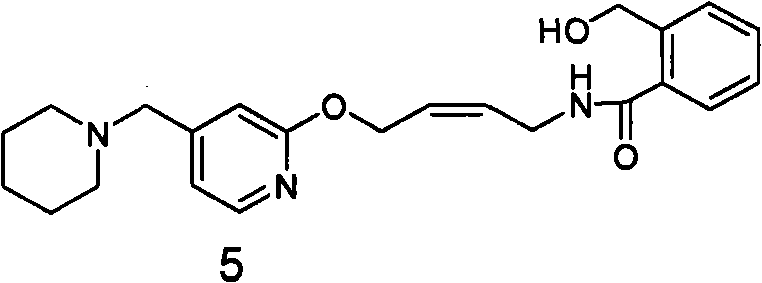
[0071] Embodiment 2: the present invention prepares lafutidine with reducing agent reduction method
[0072] 1. Preparation of 2-hydroxymethyl-N-(4-(4-piperidinylmethyl)pyridyl-2-oxo)-2-enbutyl)benzamide (compound of formula 5)
[0073]
[0074] 1600 milliliters of isopropanol and 263 milliliters of water were put into the reaction flask, and 73 grams (0.187 mol) of 2 oil was added, and the reaction liquid was clear after mechanical stirring for 5 minutes. At 25°C, 35.3 g (0.934 mol) of sodium borohydride was added in batches, and the stirring reaction was continued for 15 hours. TLC showed that the reaction of the raw materials was complete, and a large amount of white solid was generated. Take a small amount of filtration, H NMR spectrum and mass spectrum prove to be 2-hydroxymethyl-N-(4-(4-piperidinylmethyl)pyridyl-2-oxo)-2-enylbutyl)benzamide (compound of formula 5). 1 H NMR (CDCl 3 ): δ1.41-1.42(m, 2H); 1.52-1.57(m, 4H); 2.33(s, 4H); 3.36(s, 2H); 4.17-4.19(t, 2H);...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com