Novel polyethersiloxanes carrying alkoxysilyl groups and method for the production thereof
A technology of alkoxysilyl and alkoxysilyl functions, applied in the field of new polyether-siloxane with alkoxysilyl and its preparation, can solve the problem of no surfactant performance And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
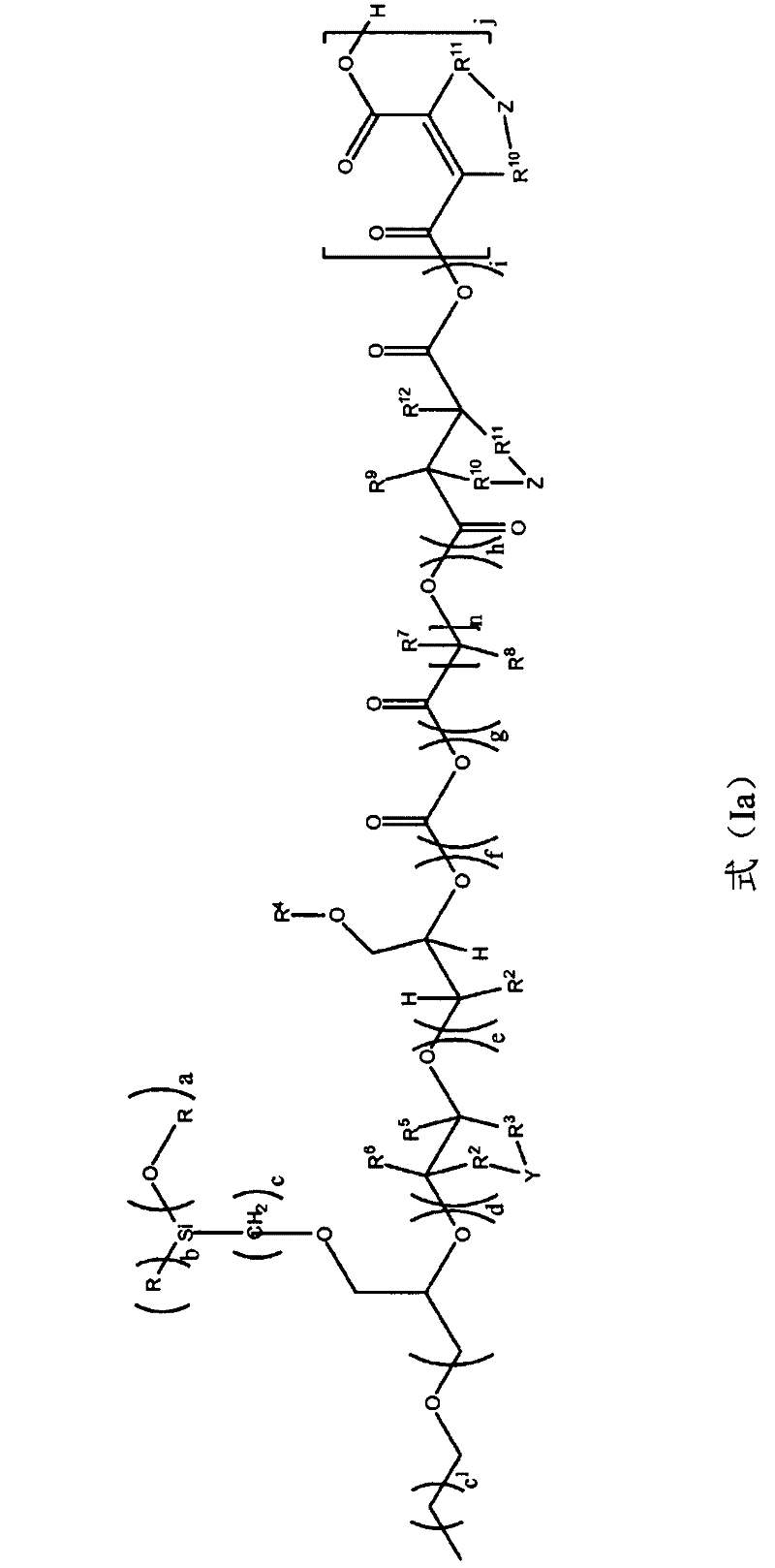
[0085] The invention also provides the following process for preparing alkoxysilyl-functionalized polyether-siloxanes, which are processed to give a siloxane backbone.
[0086] Alkoxysilyl-functionalized polyether-siloxanes and their mixtures can be prepared by two different methods:
[0087] 1) alkoxylation of polysiloxane-polyether copolymers or polysiloxanes with epoxy-functionalized alkoxysilanes using double metal cyanide catalysts,
[0088] and / or
[0089] 2) Hydrosilylation coupling of unsaturated polyethers bearing alkoxysilyl groups, which are alkane functionalized by epoxy using DMC catalysts Oxysilanes are obtained beforehand by alkoxylation of the corresponding unsaturated starter compounds.
[0090] These two methods can be carried out independently of each other, but also sequentially in a desired manner.
[0091] These two methods are described in detail below.
[0092] 1) Polysiloxane- Alkoxylation of polyether copolymers or polysiloxanes
[0093] Th...
Embodiment 11
[0200] As a chain initiator, use has the structure k=27, l 3 =5, l 4 =o=0, X=X 6 =Methyl polyether-siloxanes of the formula (IX) used in excess according to the prior art with an average molar mass of 800 g / mol and containing 64% by weight of ethylene oxide units and 36% by weight of propylene oxide OH-terminated allyl polyethers of the units, previously prepared by hydrosilylation of hydrogen siloxanes.
[0201] Under nitrogen, 550 g of this polyether-siloxane and 0.071 g of zinc hexacyanocobaltate DMC catalyst were charged to a 3-liter autoclave and heated to 130° C. with stirring. The reactor was pumped down to an internal pressure of 30 mbar, so that any volatile constituents present were removed by distillation. To activate the DMC catalyst, a 40.0 g portion of propylene oxide was added. After 15 minutes and reaction start (decrease in reactor internal pressure), 166.8 g of 3-glycidyloxypropyltriethyl was added continuously within 1 hour at 130° C. and a maximum react...
Embodiment 12
[0204] As a chain initiator, use has the structure k=25, l 3 = l 4 =o=0, X=methyl, X 6 =-(CH 2 ) 6 -OH linear polyether-siloxanes of the formula (IX), which were previously prepared by hydrosilylation of hydrogen siloxanes using hexenol according to the prior art.
[0205] Under nitrogen, 200 g of this polyether-siloxane and 0.027 g of zinc hexacyanocobaltate DMC catalyst were added to a 1 liter glass flask with a reflux condenser and heated to 130° C. with stirring. The reactor was pumped down to an internal pressure of 30 mbar, so that any volatile constituents present were removed by distillation. To activate the DMC catalyst, a first portion of 10.0 g of 3-glycidyloxypropyltriethoxysilane (DYNASYLAN GLYEO). After 15 minutes, 88.4 g of DYNASYLAN was added continuously over 1.5 hours at 130°C GLYEO. Then, 28.8 g of 1,2-epoxybutane were added within 1.5 hours at 130° C. under gentle reflux. After 1 hour post reaction all the butylene oxide had reacted and no refl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Oh value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com