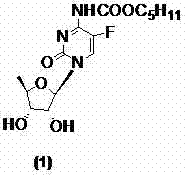
Preparation method of capectabine
A capecitabine and cytidine technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve problems such as unfavorable industrial production, and achieve easy control, high yield and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
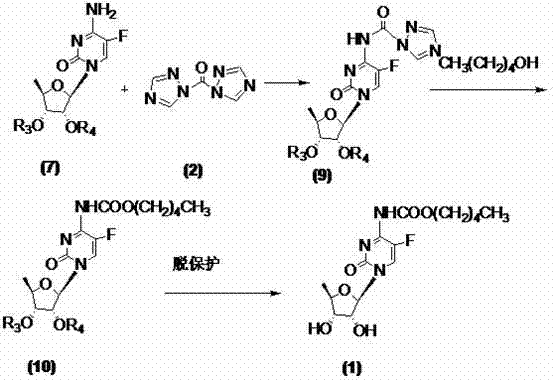
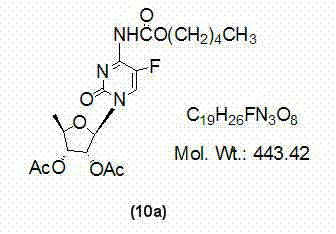
[0033] At room temperature, dissolve 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (7a) (10g, 30mmol) with 400ml of dichloromethane and add 1,1' -Carbonyl bis(1,2,4-triazole) (9.8g, 60mmol), TLC monitoring until the reaction is complete (developing solvent: dichloromethane / methanol=12:1), add n-pentanol (6.6ml, 60mmol ), TLC monitored until the reaction was complete (developing solvent: dichloromethane / methanol=12:1), washed the reaction mixture with 150ml×3 water, dried the organic phase with anhydrous sodium sulfate, filtered, and concentrated the filtrate to dryness to obtain a yellow oil , namely compound (10a) 10.7g, yield 80.5%, directly used in the next step reaction without purification.
Embodiment 2
[0034] Example 2: Preparation of 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]-cytidine nucleoside (10a).
[0035] At room temperature, dissolve 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (7a) (10g, 30mmol) with 400ml of dichloromethane and add 1,1' -Carbonyl bis(1,2,4-triazole) (13.1g, 80mmol), TLC monitoring until the reaction is complete (developing solvent: dichloromethane / methanol=12:1), add n-pentanol (6.6ml, 60mmol ), TLC monitored until the reaction was complete (developing solvent: dichloromethane / methanol=12:1), washed the reaction mixture with 50ml×3 water, dried the organic phase with anhydrous sodium sulfate, filtered, and concentrated the filtrate to dryness to obtain a yellow oil , that is, compound (10a) 11.0 g, yield 82.7%, was directly used in the next step reaction without purification.
Embodiment 3
[0037]
[0038]The 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]-cytidine (10a) obtained in Example 1 (8.9g, 20mmol), 150ml of methanol was stirred and dissolved, at -25℃~-20℃, 50ml of 2mol / L sodium hydroxide solution was added dropwise, and TLC monitored until the reaction was complete ((developing solvent: dichloromethane / methanol=9:1), Adjust the pH to 5.0-6.0 with concentrated hydrochloric acid, extract with 200ml×3 dichloromethane, combine the organic phases, dry with anhydrous sodium sulfate, filter, concentrate the filtrate to dryness, and recrystallize from ethyl acetate to obtain a white solid, the target compound card Betabine (1) 6.2g, yield 86.1%, HPLC: 99.97%, melting point: 113-116°C, specific rotation: 98.10°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com