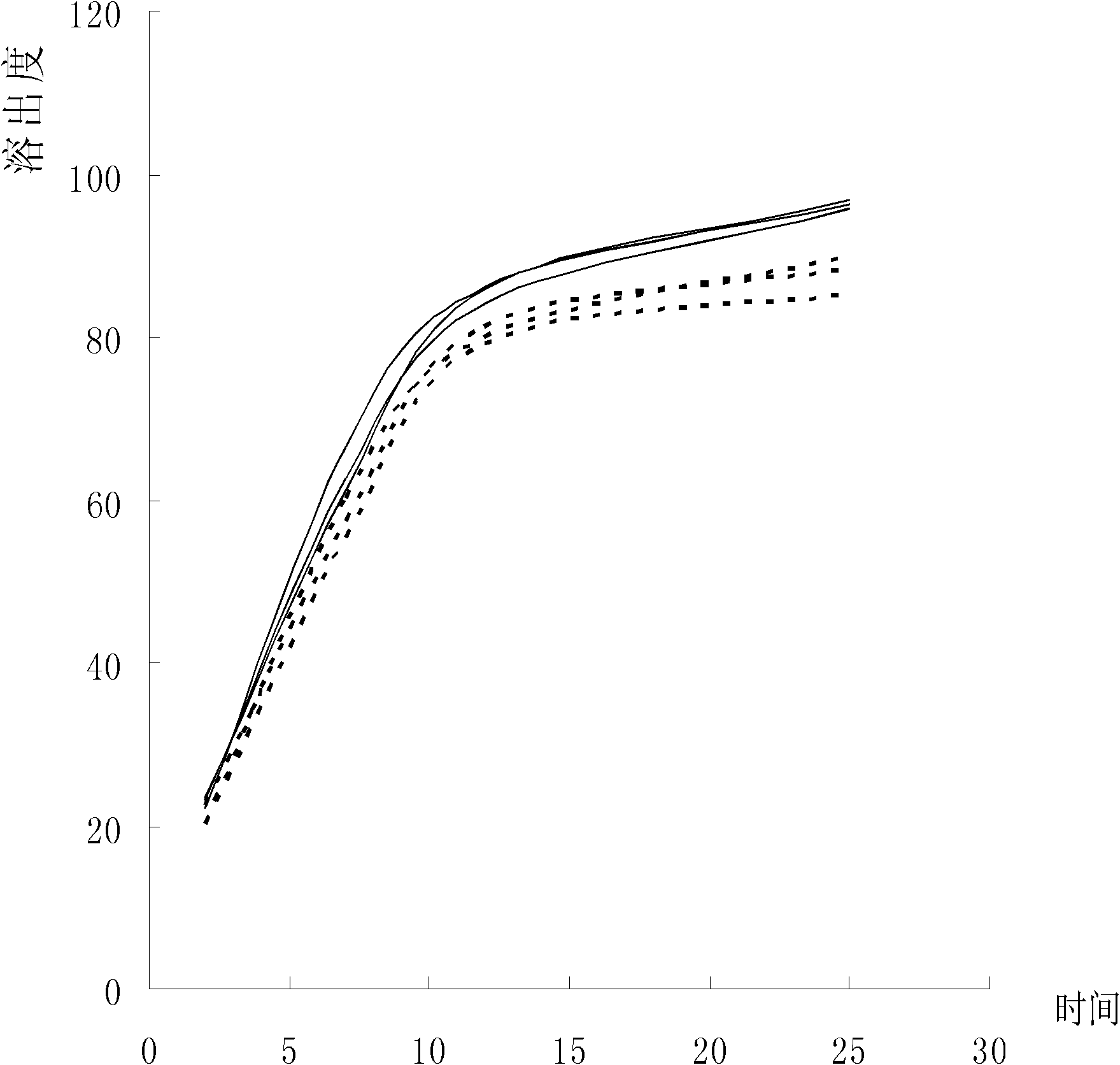
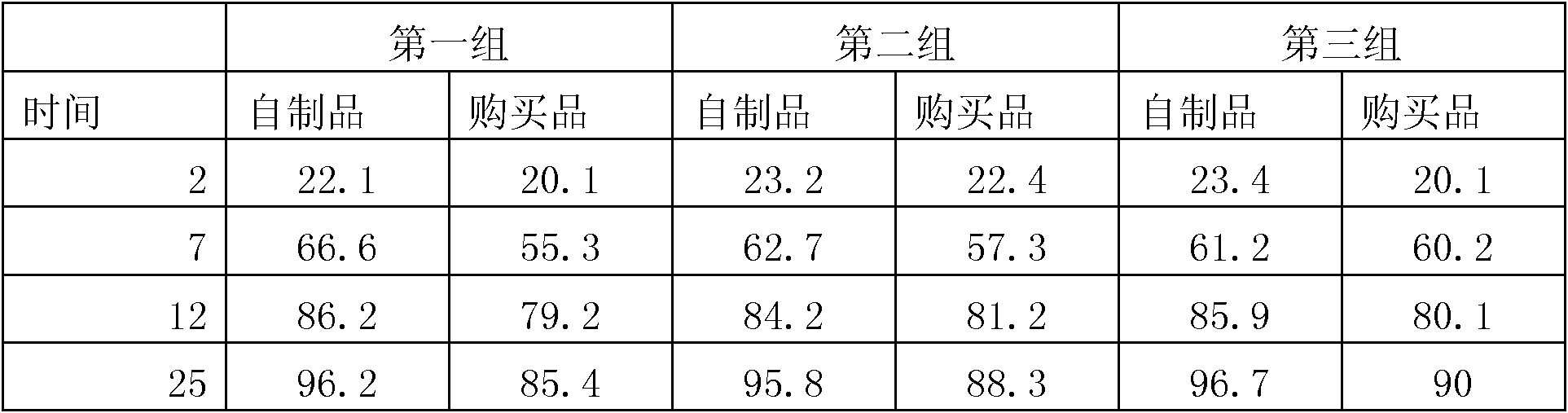
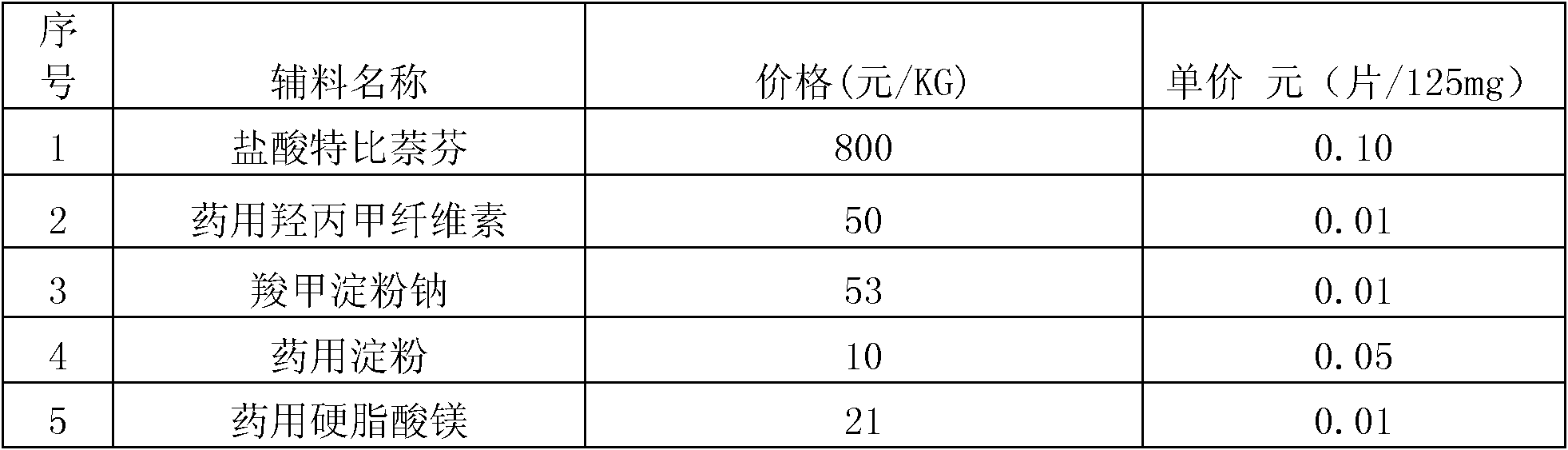
Preparation method of antifungal tablet
A technology for antifungal drugs and tablets, which is applied in the field of preparation of antifungal drugs, can solve the problems of improper addition of excipients, disintegration time, difficult quality control, low dissolution limit, etc., and achieve strong effect on superficial fungal infection and good formability Good quality stability and oral absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0011] Specific embodiment one: the preparation method of the antifungal drug tablet of the present embodiment is carried out according to the following steps: one, take by weight 100~150 parts of terbinafine hydrochloride, 4~8 parts of binder, 8 parts by weight ~15 parts of carboxymethyl starch sodium, 50~60 parts of starch and 0.5~3 parts of magnesium stearate; 2. Place the weighed terbinafine hydrochloride, starch and 6~10 parts of carboxymethyl starch sodium Mix in a mixing tank for 20 minutes, then add binders to mix to make soft materials, pass through a 14-mesh sieve for granulation, pass through a 14-mesh sieve for granulation after drying, add the weighed magnesium stearate and the remaining amount of carboxymethyl starch sodium, After mixing and pressing into tablets, the preparation of antifungal drug tablets is completed; in step 1, the binder consists of hypromellose solution with a mass concentration of 1% to 10% and starch slurry with a mass concentration of 4% t...
specific Embodiment approach 2
[0014] Specific embodiment two: What this embodiment differs from specific embodiment one is that in step one, take by weight 100 parts of terbinafine hydrochloride, 4 parts of adhesive, 8 parts of sodium starch glycolate, 50 parts of starch and 0.5 parts of magnesium stearate. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0015] Specific embodiment three: the difference between this embodiment and specific embodiment one is that in step one, take by weight 150 parts of terbinafine hydrochloride, 8 parts of adhesive, 15 parts of sodium starch glycolate, 60 parts starch and 3 parts magnesium stearate. Other steps and parameters are the same as those in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com