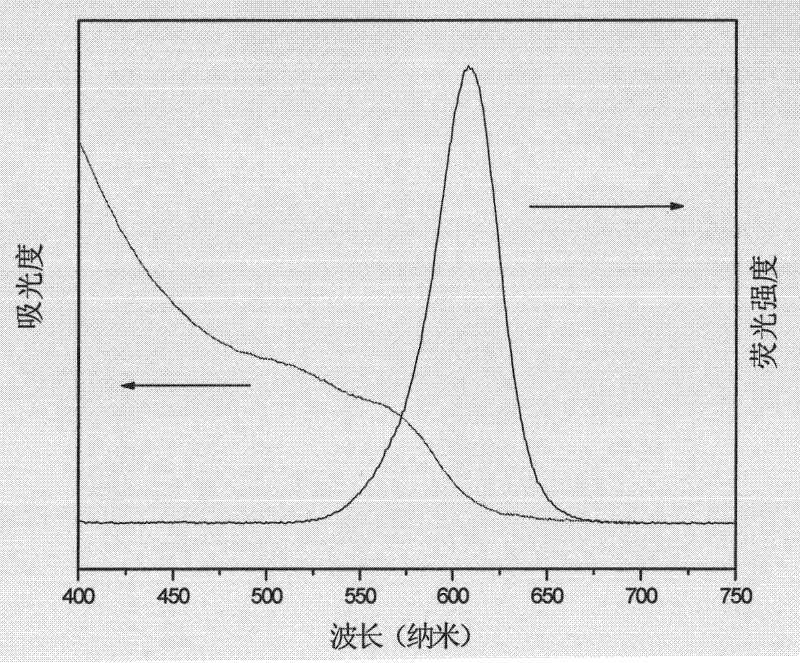
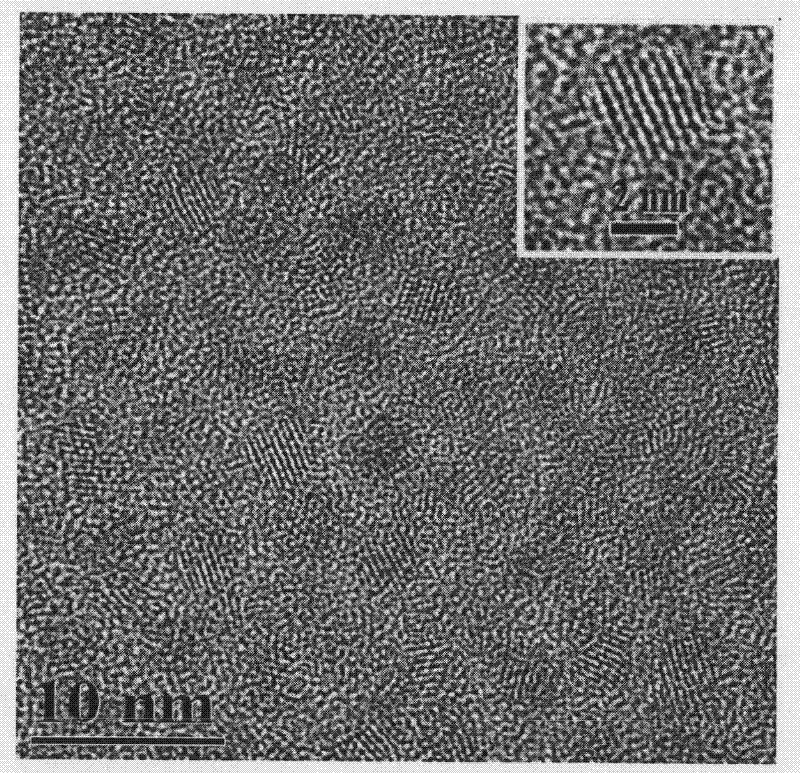
Method for preparing CdTe quantum dots in aqueous phase and at low temperature
A technology in quantum dots and water phase, which is applied in the field of nanomaterial preparation, can solve the problems of reducing the fluorescence yield of quantum dots, demanding the preparation process, and the high price of reagent toxicity, and achieves low requirements for reaction equipment, good dispersion, and high preparation efficiency. convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (a) Ligand Preparation
[0029] Add 5g proline, 3.47g NaOH, 50ml deionized water into a 100ml flask, stir for 30min, and dissolve 2.76mlCS 2 Dissolve it in 25ml of ethanol, slowly drop it into the flask, and finish adding in 50 minutes. After 24 h of reaction, the solvent was removed under reduced pressure.
[0030] (b) Preparation of cadmium source
[0031] Add CdCl to the 2L flask 2 229mg, 1L of ultrapure water, 440mg of proline dithiocarbamate sodium salt, adding NaOH solution to adjust the pH of the solution to 8 to obtain a cadmium source of 1.25mmol / L.
[0032] (c) Preparation of tellurium source
[0033] Add 95.7mg Te powder, 73mg NaBH4, 3ml ultrapure water into a vial with an air outlet, and react at 0°C for 24 hours to obtain a 250mmol / L tellurium source. .
[0034] (d) Growth of CdTe
[0035] Pass high-purity nitrogen gas through 1L of 1.25mmol / L cadmium source solution for 30min, inject 1ml of freshly prepared tellurium source, and react in a water ba...
Embodiment 2
[0037] (a) Ligand Preparation
[0038] Add 5g proline, 3.47g NaOH, 50ml deionized water into a 100ml flask, stir for 30min, and dissolve 3.3ml CS 2 Dissolve it in 40ml of ethanol, slowly drop it into the flask, and finish adding in 30 minutes. After 24 h of reaction, the solvent was removed under reduced pressure.
[0039] (b) Preparation of cadmium source
[0040] Add CdCl to 1L flask 2 229mg, 500ml of ultrapure water, 586mg of proline dithiocarbamate sodium salt, adding NaOH solution to adjust the pH of the solution to 9 to obtain a cadmium source of 2.5mmol / L. .
[0041] (c) Preparation of tellurium source
[0042] Add 95.7mg of Te powder, 73mg of NaBH4, 3ml of ultrapure water into a vial with an air outlet, and react at 10°C for 15 hours to obtain a 250mmol / L tellurium source.
[0043] (d) Growth of CdTe
[0044] Pass high-purity nitrogen gas through 1L of 2.5mmol / L cadmium source solution for 30min, inject 3ml of freshly prepared tellurium source, and react in a w...
Embodiment 3
[0046] (a) Ligand Preparation
[0047] Add 5g of proline, 3.47g of NaOH, and 50ml of deionized water into a 100ml flask, stir for 30min, dissolve 5.52ml of CS2 in 25ml of ethanol, slowly drop it into the flask, and complete the addition within 20min. After 24 h of reaction, the solvent was removed under reduced pressure.
[0048] (b) Preparation of cadmium source
[0049] Add CdCl to 1L flask 2 229mg, 1L of ultrapure water, 440mg of proline dithiocarbamate sodium salt, adding NaOH solution to adjust the pH of the solution to 10 to obtain a cadmium source of 1.25mmol / L.
[0050] (c) Preparation of tellurium source
[0051] Add 95.7mg Te powder, 110.5mg KBH4, and 3ml ultrapure water into a vial with an air outlet, and react at 0°C for 20 hours to obtain a 250mmol / L tellurium source.
[0052] (d) Growth of CdTe
[0053] Pass high-purity nitrogen gas through 1L of 1.25mmol / L cadmium source solution for 30min, inject 1ml of freshly prepared tellurium source, and react in a 50°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com