Micro-fluidic chip for in-vivo on-line simultaneous detection of ascorbic acid and magnesium ion and preparation method thereof
A microfluidic chip and liquid flow technology, which is applied in diagnostic recording/measurement, medical science, instruments, etc., can solve the problems of immaturity and late start of microfluidic chip technology, so as to improve the analysis speed and consume samples and reagent electrode, liquid flow controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
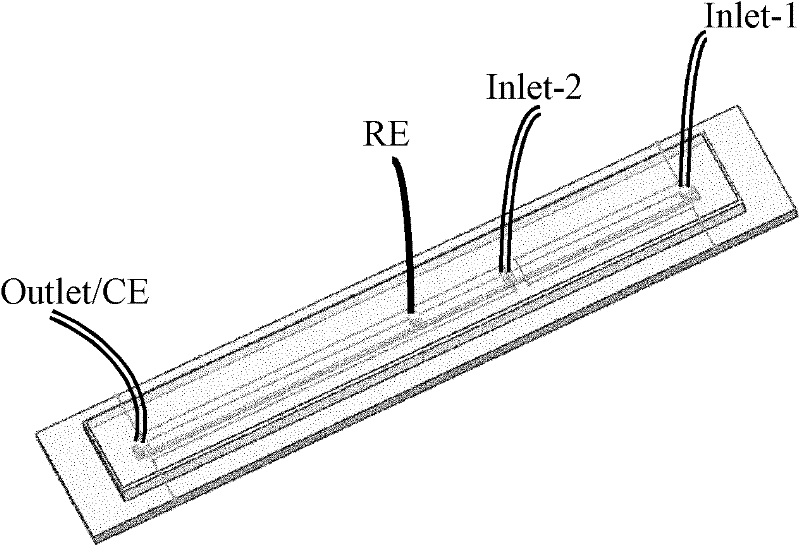
[0030] Example 1. Preparation of a microfluidic chip capable of simultaneously detecting ascorbic acid and magnesium ions in vivo
[0031] 1) In 6×6cm 2 A "one"-shaped channel is designed in the center of the plexiglass, and its length, width and height are 4cm, 1mm, and 500μm, respectively. A polydimethylsiloxane template with a height of 1 cm was obtained after polydimethylsiloxane was molded twice, and it was cut into a rectangle with a length of 5 cm and a width of 1.5 cm without affecting the use of the channel.
[0032]2) Corrode the indium tin oxide conductive glass with a length of 6 cm and a width of 1.5 cm into two independent centered rectangular base electrodes in the same direction as its length. The electrodes are 1.5 cm and 2.5 cm in length and 0.5 cm in width.
[0033] Firstly, toluidine blue was electropolymerized on a 2.5 cm long substrate electrode, and in a 100 μM toluidine blue solution dissolved in a phosphate buffer solution with a pH of 7, at a scannin...
Embodiment 2
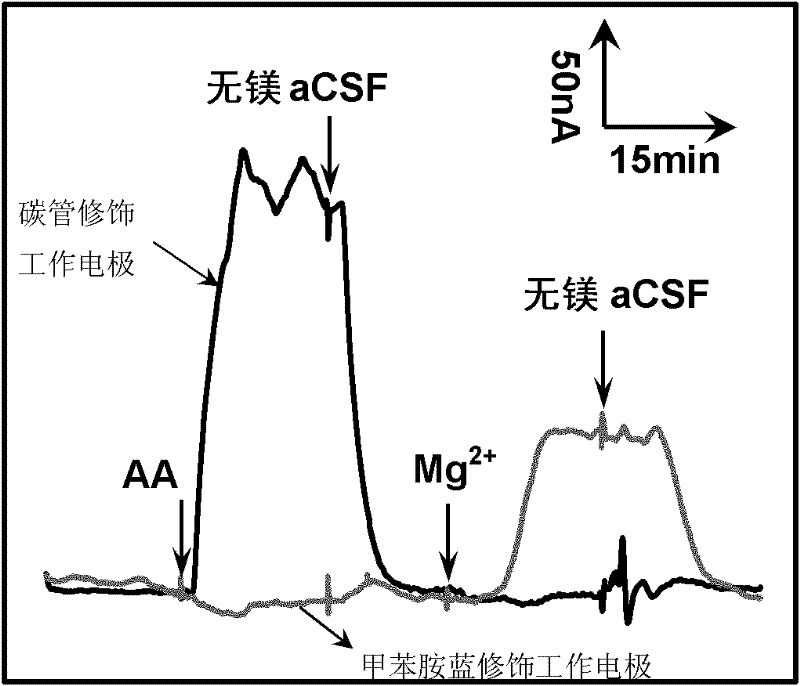
[0038] Example 2. In vitro cross-interference experiment of a microfluidic chip capable of simultaneously detecting ascorbic acid and magnesium ions in vivo
[0039] Under the premise that the liquid flow rate is fixed at 1.0 μL / min, the secondary liquid inflow inlet is continuously fed with β-nicotinamide adenine dinucleotide (abbreviated as NADH) solution (first, NADH is used as a system for detecting magnesium ions. It must exist, and secondly, it has interference in the detection system of ascorbic acid and must be eliminated. Therefore, NADH is a substance that must exist in the downstream detection system but interferes with the upstream detection system and should be excluded. Substances like NADH in the sub-detection can be designed with a secondary liquid flow inlet and sampled from the secondary liquid flow inlet. This can not only ensure the detection of the downstream system, but also eliminate its interference in the upstream system, so as to realize the two-compon...
Embodiment 3
[0044] Example 3. In vitro quantitative detection experiment of a microfluidic chip capable of simultaneously detecting ascorbic acid and magnesium ions in vivo
[0045] Under the premise that the liquid flow rate was fixed at 1.0 μL / min, the NADH solution was continuously fed into the secondary liquid inlet, and the main liquid inlet was mixed with magnesium-free aCSF and different concentrations (mixtures of different concentrations of ascorbic acid and magnesium ions). liquid) between alternate injections.
[0046] image 3 It shows the time-current curves of the microfluidic chip detecting different concentrations of ascorbic acid and magnesium ions in vitro. The black line is the I-t curve of the upstream carbon tube modified working electrode (measurement voltage 0.03V), and the red line is the downstream toluidine blue modification. The I-t curve of the electrode (measurement voltage 0V). The secondary liquid inflow port was fed with 2mM NADH solution continuously, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com