Cefathiamidine hydrate and preparation method and application thereof
A technology of cefathiamidine and hydrate, which is applied in the field of medicine, can solve the problems such as the preparation method and use of cefathiamidine crystal hydrate that are not reported in the literature, and achieves improved operability, good sliding property, and convenient storage and transportation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of Cefathiamidine 0.25 Hydrate Suspend 8 g of 7-bromoacetyl ACA in 160 ml of dichloromethane, add 3 ml of triethylamine, stir to dissolve completely, add 0.2 g of activated carbon and stir for 30 minutes, filter, filter Wash the cake with 10ml of dichloromethane, combine the filtrate and washing liquid, add 3.8g of N,N'-diisopropylthiourea, stir, react at 35-40°C for 1.5 hours, cool to below 15°C, and slowly add Acetone 200ml, chloroform 20ml, stand for 3 hours, filter with suction, soak the crystals with a small amount of chloroform and acetone 3 times, filter with suction, dissolve the obtained solid in 10ml of water, slowly add 200ml of ethanol, 10ml of acetonitrile for recrystallization, 0℃ Place it below until the precipitate is fully separated, filter it to dryness, and dry the solid at about 40 ° C for about 5 hours to obtain 3.3 g of off-white crystals, melting point: 155 ° C decomposition (ELECTROTHERMAL MELTING POINT APPARATUS, uncorrected...
Embodiment 2
[0056] Example 2 Preparation of Cefathiamidine 0.25 Hydrate Suspend 8 g of 7-bromoacetyl ACA in 180 ml of dichloromethane, add 3.2 ml of diisopropylamine, stir to dissolve completely, add 0.2 g of activated carbon and stir for 25 minutes, filter, Wash the filter cake with 10ml of chloroform, combine the filtrate and washing liquid, add 3.8g of N,N'-diisopropylthiourea to it, stir, react at 35-40°C for 1.5 hours, cool to below 10°C, and slowly add acetone 200ml, 20ml ethyl acetate, stand for 3 hours, filter with suction, soak the crystals with a small amount of acetone and acetonitrile for 3 times, filter with suction, dissolve the obtained solid in 10ml of water, slowly add 20ml of ethanol and 120ml of ether for recrystallization, 0 Place below ℃, wait until the precipitate is fully separated, filter to dryness, and dry the solid at about 35℃ for about 5 hours to obtain 2.1g of off-white crystals, melting point: 155℃ decomposition (ELECTROTHERMAL MELTING POINT APPARATUS, uncorr...
Embodiment 3
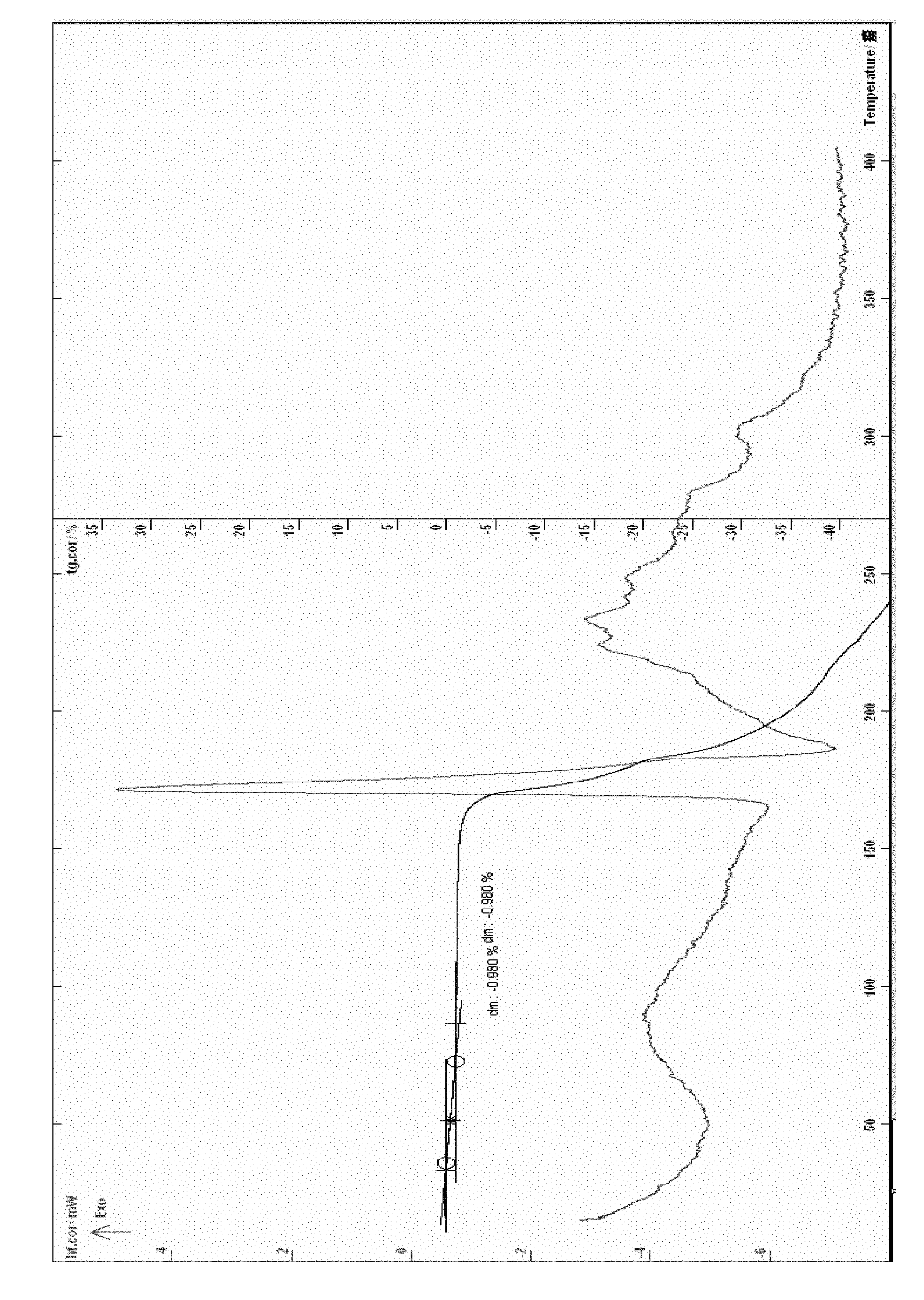
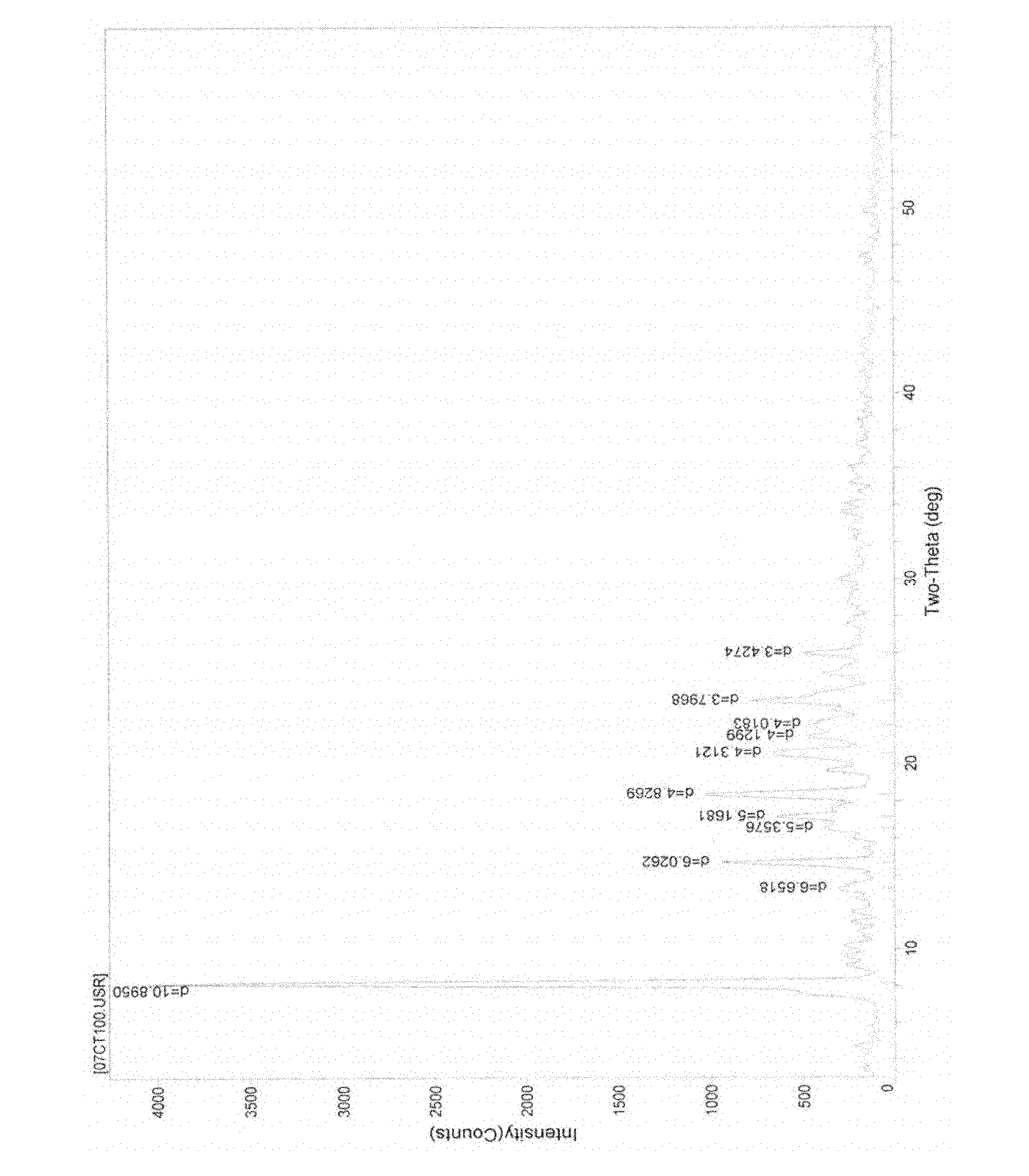
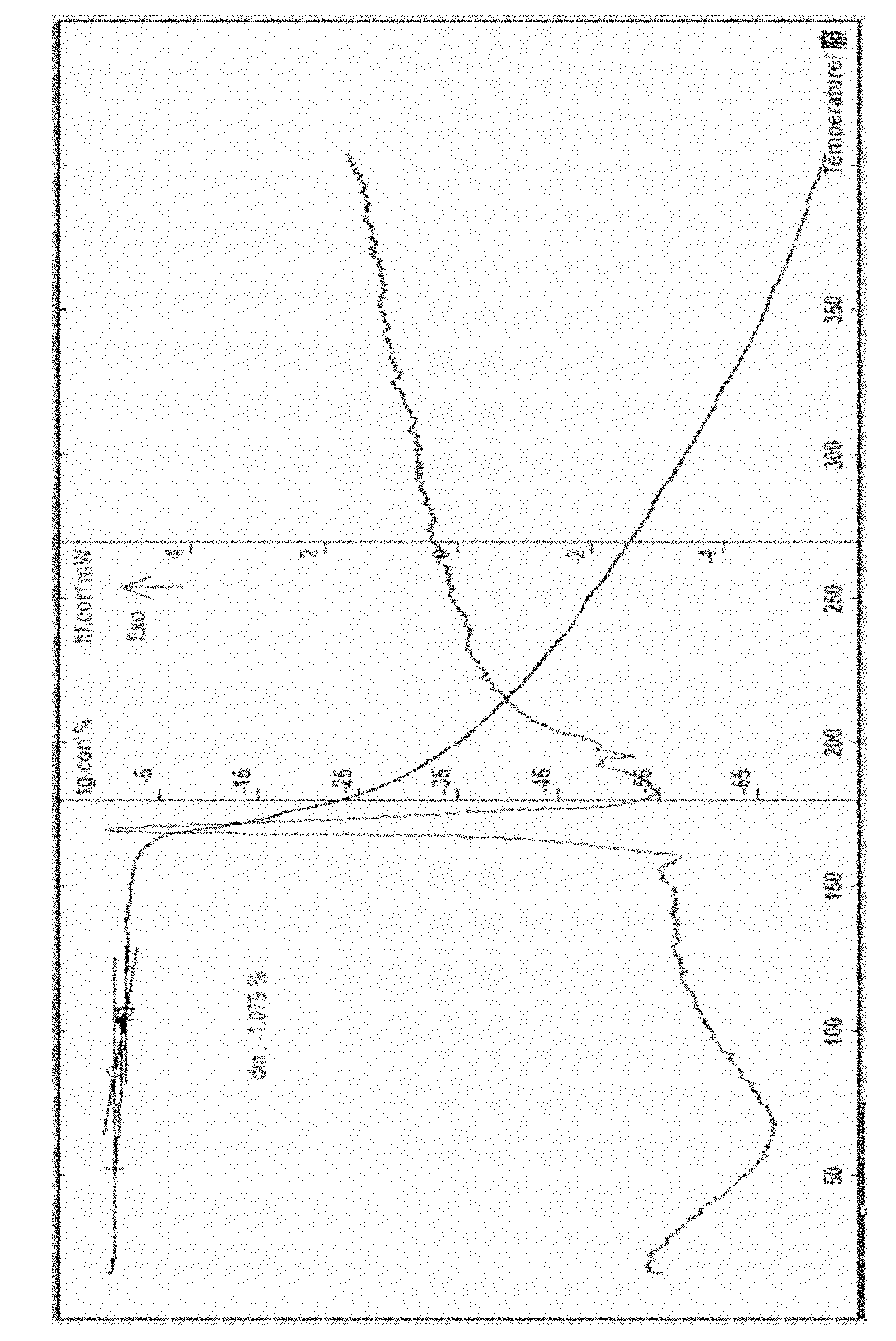
[0058] Example 3 Put 5 g of cefathiamidine in a container and add 10 ml of water to dissolve it, put the solution in a freeze-drying box, lower the temperature of the liquid to -40°C, and pre-freeze for 4 hours; lower the temperature of the condenser to -45°C, Vacuumize, control the degree of vacuum and control the temperature of the plate layer to rise below -15°C, freeze and sublimate, and sublimate and dry for about 17 hours; continue heating to gradually increase the temperature to about 30°C at a rate of 10°C per hour, and keep warm for about 3 hours. Close the hydrazine butterfly valve of the box for 3 minutes, and when the pressure of the box rises to within 5Pa, the entire freeze-drying process is ended, and the gas is released from the box to obtain cefathiamidine 0.5 hydrate; [α] D 20 : +140; Karl Fischer's method of measuring moisture is 1.96%, thermal analysis: platform weight loss of about 1.80%, which is within the error range with the result of the sample contai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com