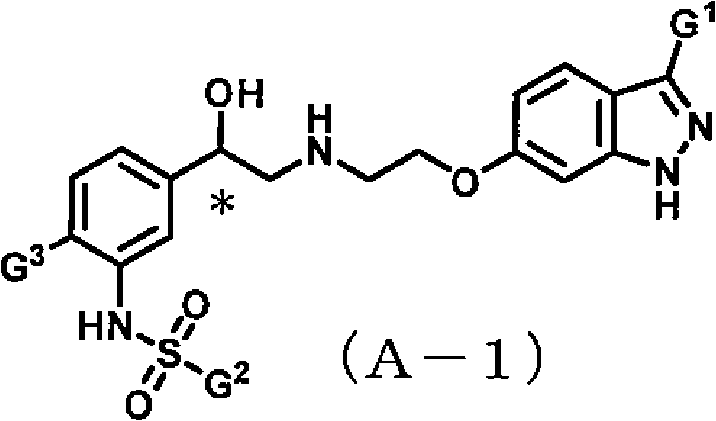
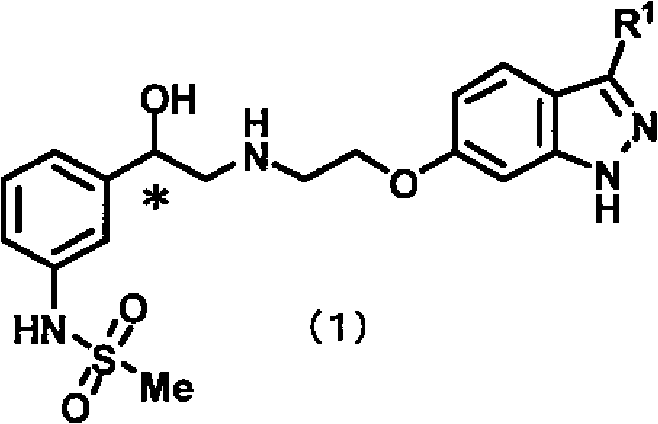
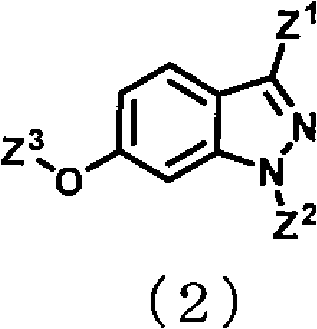
Indazole compound
A compound, the technology of ethylindazole, applied in the direction of active ingredients of silicon compounds, organic chemistry, drug combination, etc., can solve the problems such as no suggestion, no disclosure of β3 adrenergic receptor selective stimulation, no teaching, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0613] The present invention will be further described based on examples, reference examples, and test examples, but the scope of the present invention is not limited to the following examples.
[0614] In the following examples, various analyzes were performed as follows.
[0615] (1) Precoated silica gel 60F254 (manufactured by Merck, product number 5715-1M) was used for thin layer chromatography (TLC). After developing with chloroform:methanol (1:0~1:1) or ethyl acetate:hexane (1:0~0:1), irradiate with UV (254nm or 365nm), pass iodine solution, potassium permanganate Confirm the color development of aqueous solution, phosphomolybdic acid (ethanol solution), ninhydrin or dinitrophenylhydrazine hydrochloric acid solution.
[0616] (2) Column chromatography was performed by the following method.
[0617] For the case described as "COLUMN-A", use Multi Prep YFLC (manufactured by Yamazensha), and use Hi-Flash made by the company for the column TM Column-Silicagel series.
...
Embodiment 6
[0664]
Embodiment 19
[0666]
[0667] (R)-N-(2-fluoro-5-(2-iodo-1-(triethylsilyloxy)ethyl)phenyl)methylsulfonamide: International Publication Patent No. WO97 / 25311 ( Cited here) Intermediate 101
[0668]
[0669] (R)-N-(2-Chloro-5-(2-iodo-1-(triethylsilyloxy)ethyl)phenyl)methylsulfonamide: International Patent No. WO97 / 25311 Intermediate 107
[0670]
[0671] 1-Benzyl-3-methylindazol-6-ol: Reference Example 11 of International Laid-Open Patent No. WO03 / 035620
[0672]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com