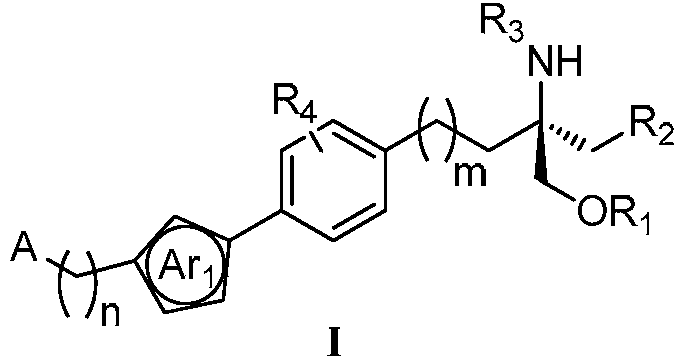
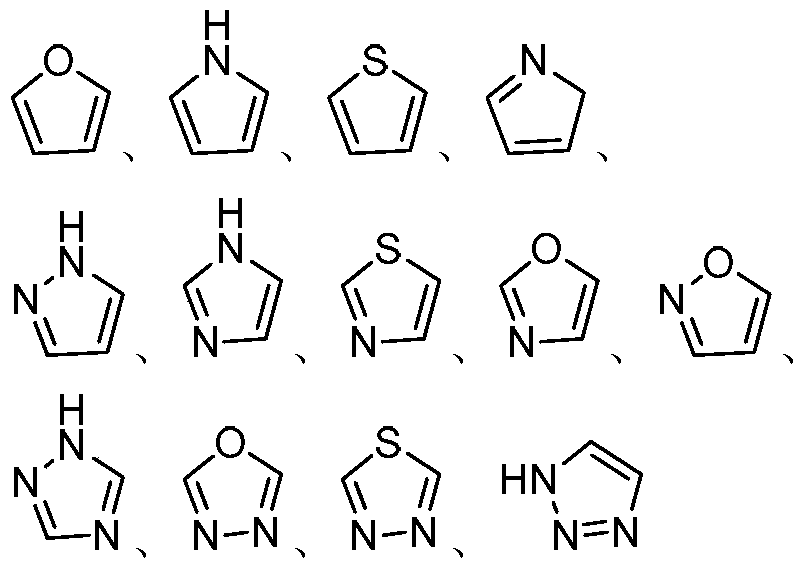
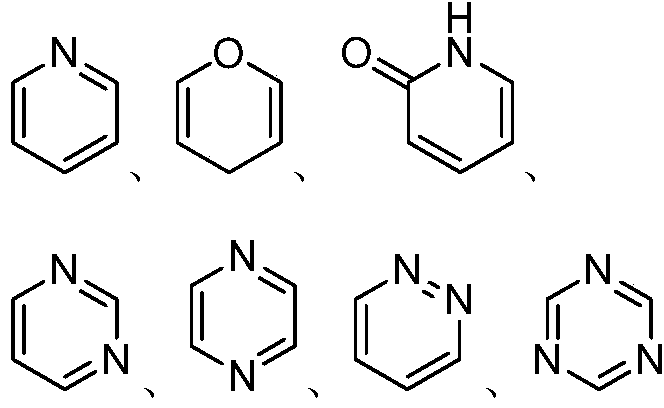
Aminopropanediol compound containing five-membered aromatic heterocycle, its preparation method and its medical application
A compound, amino technology, applied in the field of medicinal chemistry, can solve problems such as blood pressure lowering, bradycardia, etc., and achieve the effect of high immunosuppressive activity in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] 2-Amino-2-(4-(2-pentyloxazol-4-yl)phenethyl)propane-1,3-diol hydrochloride
[0128]
[0129] (1) Preparation of 2-acetylamino-2-phenylethylmalonate
[0130]
[0131] Sodium metal (4.1g, 178.3mmol) was dissolved in 250mL of absolute ethanol, diethyl acetamidomalonate (38.7g, 178.3mmol) was added in batches under ice cooling, and stirred at room temperature for 2 hours. Add 2-bromophenylethane (30g, 162.1mmol) in anhydrous ethanol solution dropwise under ice bath, reflux at 80°C for 18 hours, filter, concentrate the filtrate, dissolve the residue in 200ml of ethyl acetate, wash with saturated ammonium chloride solution 1 time, washed 2 times with water, 1 time with saturated sodium chloride solution, and dried over anhydrous sodium sulfate. Concentrate and separate by column chromatography, the eluent is petroleum ether: ethyl acetate = 3:1, to obtain 23 g of white solid, the yield is 44.2%. The melting point is 112-115°C.
[0132] 1HNMR (300MHz, CDCl 3 )δ7.29-7...
Embodiment 2
[0159] 2-amino-2-(4-(1-pentyl-1H-1,2,3-triazol-4-yl)phenethyl)propane-1,3-diol hydrochloride
[0160]
[0161] (1) Preparation of 2-acetylamino-2-(4-bromophenethyl)diethyl malonate
[0162]
[0163]Sodium metal (3.66g, 159.1mmol) was dissolved in 250mL of absolute ethanol, and diethyl acetamidomalonate (36.2g, 166.7mmol) was added in batches under ice-cooling, and stirred at room temperature for 2 hours. Add 1-bromo-4-(2-bromoethyl)benzene (40g, 151.5mmol) in anhydrous ethanol solution dropwise under ice bath, reflux at 80°C for 18 hours, filter, concentrate the filtrate, add 300ml ethyl acetate to the residue dissolved, washed once with saturated ammonium chloride solution, twice with water, once with saturated sodium chloride solution, and dried over anhydrous sodium sulfate. Concentrate and separate by column chromatography, the eluent is petroleum ether: ethyl acetate = 3:1, to obtain 20 g of a yellow solid, with a yield of 32.8%. The melting point is 35-38°C.
[...
Embodiment 3
[0191] 2-Amino-2-[4-(1-(4-methylbenzyl)-1H-1,2,3-triazol-4-yl)phenethyl]propane-1,3-diolate salt
[0192]
[0193] (1) 2-Acetamido-2-[4-(1-(4-methylbenzyl)-1H-1,2,3-triazol-4-yl)phenethyl]propane-1,3 -diol
[0194]
[0195] Prepared according to the method of step (5) in Example 2, substituting p-methylbenzyl bromide for 1-bromo-n-pentane to obtain 187 mg of light yellow solid with a yield of 75%. Melting point 100-103°C.
[0196] 1HNMR (400MHz, CD 3 OD)δ8.15(s,1H),7.63(d,J=7.6Hz,2H),7.22-7.19(m,4H),7.14(d,J=7.6Hz,2H),5.50(s,2H) ,3.73(d,J=11.2Hz,2H),3.64(d,J=11.2Hz,2H),2.57(t,J=8.4Hz,2H),2.27(s,3H),1.98-1.92(m, 5H)
[0197] MS(ESI)m / z409.2314(M+H) +
[0198] (2) Preparation of title compound
[0199] It was prepared according to the method of step (6) in Example 1 to obtain 157 mg of a tan solid with a yield of 85.0%. The melting point is 163-167°C.
[0200] 1HNMR (400MHz, CD 3 OD)δ8.21(s,1H),7.65(d,J=8Hz,2H),7.24(d,J=8Hz,2H),7.19(d,J=7.6Hz,2H),7.13(d,J =8Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com