Preparation method of oxacillin sodium and oxacillin sodium for injection
A technology for synthesizing oxacillin sodium and oxacillin is applied in the preparation of oxacillin sodium and oxacillin sodium for injection, and in the field of oxacillin sodium and oxacillin sodium for injection products, and achieves high stability The effect of stability, high purity and stability, and stable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Synthesis of Oxacillin Sodium.
[0047] condensation
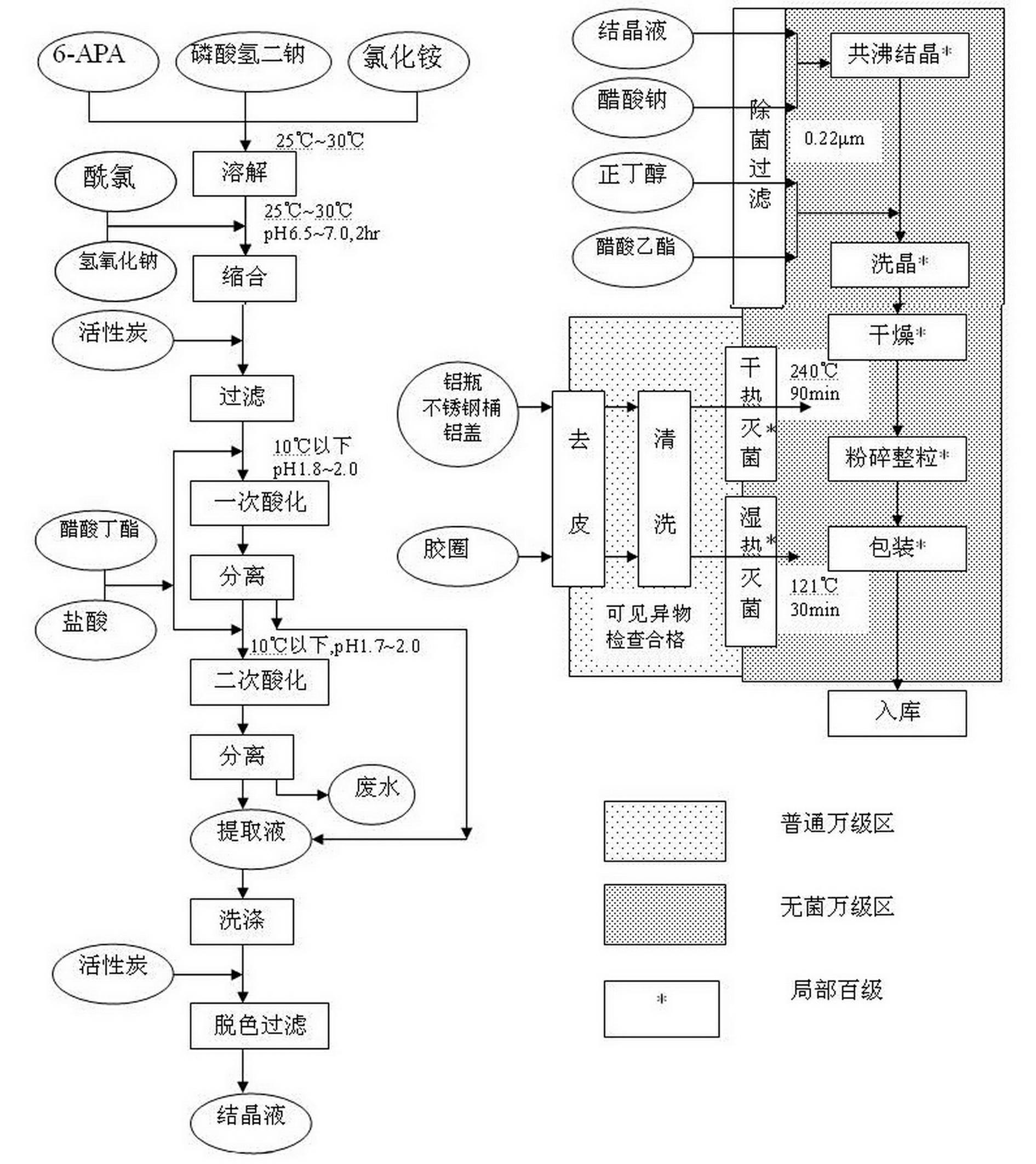
[0048] Add 4200L of purified water to the condensation tank, control the temperature to 26°C~27°C, and put in 17.5kg Na 2 HPO 4 and 1.65kg NH 4 Cl was dissolved, and 93kg of 6-APA and 100kg of acid chloride were added. Add 6mol / L NaOH solution to adjust the pH to 6.5~7.0, and react for 2 hours. Control the reaction process temperature at 25°C~28°C, pH6.5~7.0. After the reaction is over, add 3 kg of medicinal activated carbon, and filter the clarified solution after 5 to 10 minutes and add it to the primary acidification tank.
[0049] acidified extraction
[0050] Primary acidification: Add 1100~1200L butyl acetate to the primary acidification tank, add all the condensation filtrate, control the temperature at 10~12°C, and start adding 6mol / LH 2 SO 4 , adjust the pH to 1.8~2.0, and after standing for 30 minutes, add the lower aqueous phase into the secondary acidification tank.
[0051] Seco...
Embodiment 2
[0060] Example 2 Synthesis of Oxacillin Sodium.
[0061] condensation
[0062] Add 4200L of purified water to the condensation tank, control the temperature to 27°C~28°C, and put in 17.5kg Na 2 HPO 4 and 1.65kg NH 4 Cl was dissolved, and 93kg of 6-APA and 100kg of acid chloride were added. Add 6mol / L NaOH solution to adjust the pH to 6.5~7.0, and react for 2 hours. Control the reaction process temperature at 28°C~30°C, pH6.5~7.0. After the reaction is over, add 3 kg of medicinal activated carbon, and filter the clarified solution after 5 to 10 minutes and add it to the primary acidification tank.
[0063] acidified extraction
[0064] Primary acidification: Add 1100~1200L butyl acetate to the primary acidification tank, add all the condensation filtrate, control the temperature at 12~15°C, and start adding 6mol / LH 2 SO 4 , adjust the pH to 1.8~2.0, and after standing for 30 minutes, add the lower aqueous phase into the secondary acidification tank.
[0065] Seco...
Embodiment 3
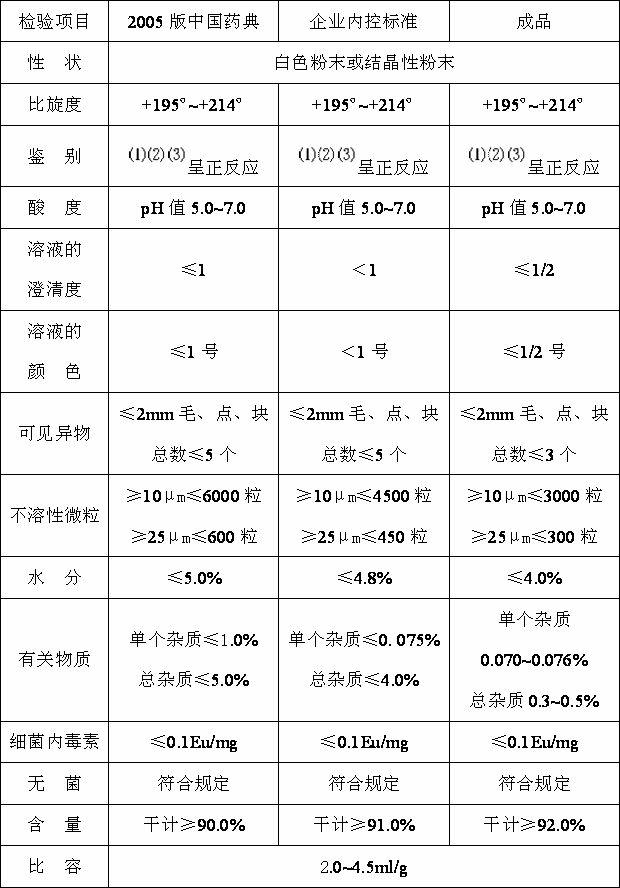
[0074] Embodiment 3 Detection of product quality.
[0075] Utilize the routine detection method that detects semi-synthetic penicillins, the products of each 3 batches that embodiment 1 and embodiment 2 make are detected, and average yield reaches 93.0%, and quality detection result is as follows:
[0076]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com