Fluorescence quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection method for rabbit hemorrhagic disease virus
A rabbit hemorrhagic disease virus, fluorescent quantitative technology, applied in the field of rabbit hemorrhagic disease virus fluorescent quantitative RT-PCR detection, molecular detection of rabbit hemorrhagic disease virus, can solve the problem that the result judgment is easily affected by various factors, poor repeatability, processing Time-consuming and other problems, to achieve the effect of avoiding false positive results, easy to operate, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 positive standard
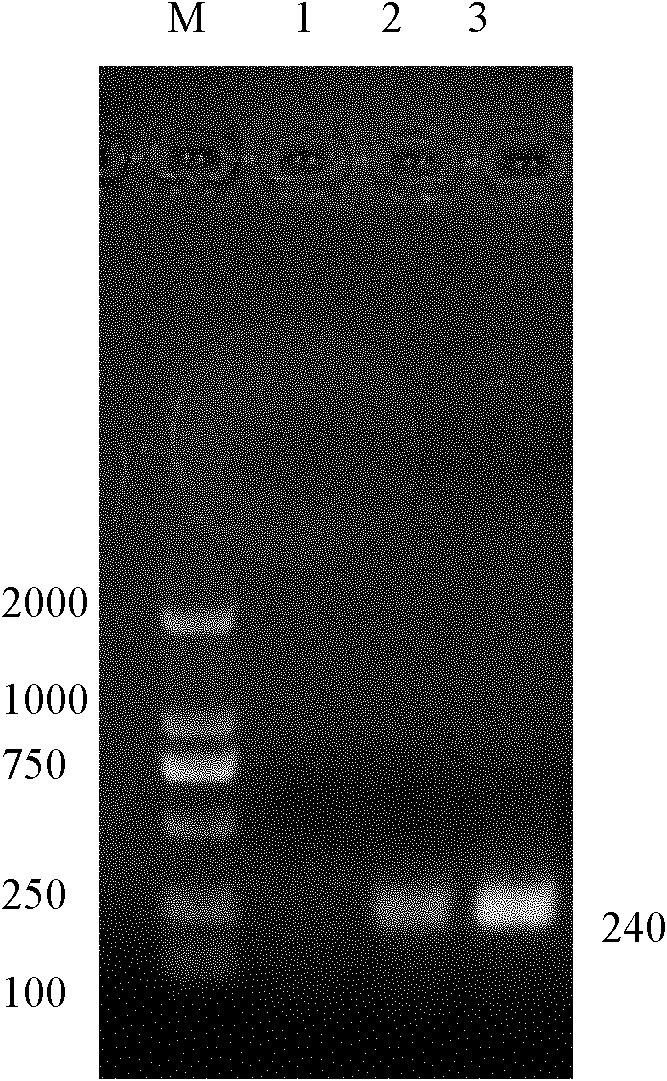
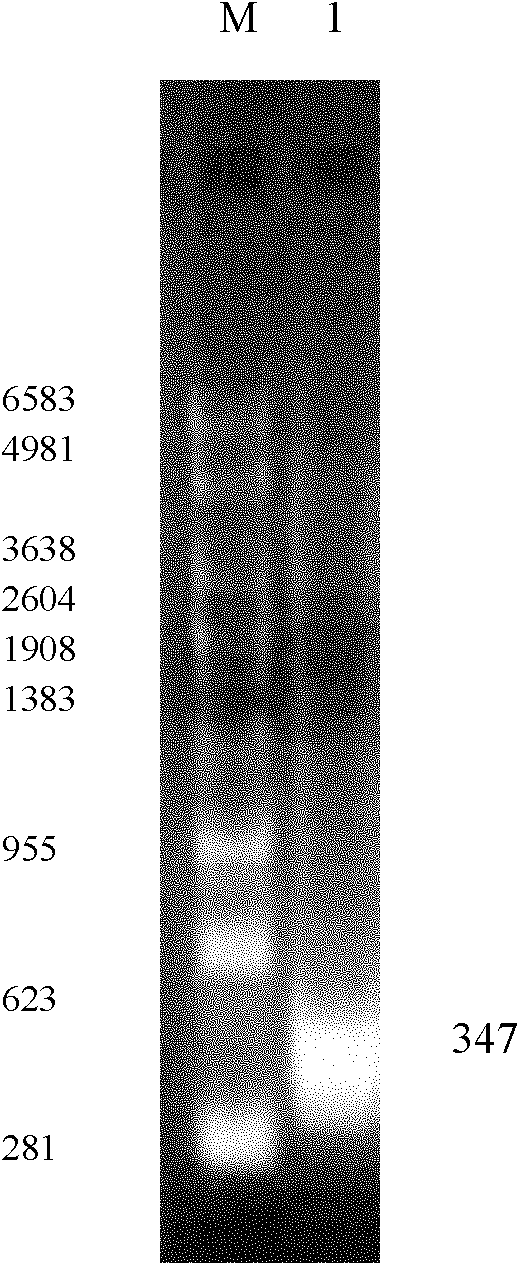
[0043] 1) RHDV virus extracts RNA, uses nucleotide sequence as the primer shown in SEQ ID No.4 and 5, carries out one-step RT-PCR amplification, obtains 240bp object fragment (such as figure 1 shown);
[0044] The PCR reaction system is:
[0045] Each 25 μL reaction solution contains: 10×RT-PCR buffer 2.5 μL, ultrapure dNTP mixture (10 mM each) 1 μL, 5×RT-PCR enhancer 5 μL, 40U / μL RNase inhibitor 0.25 μL, 2.5U / μL Hotmaster DNA polymerase 1.25 μL, Quant reverse transcriptase 0.25 μL, 10 mmol / L upstream and downstream primers 0.5 μL each, RNA template 1 μL, RNase-freeddH 2 O supplemented to 25 μL;
[0046] The PCR reaction conditions are: 50°C, 30min, 94°C, 2min, 94°C, 1min, 51.7°C, 1min, 65°C, 1min, 35 cycles of amplification, 65°C, 10min;
[0047] 2) The target fragment was then cloned into the PGEM-T Easy vector (purchased from Promega), and transformed into the host cell Escherichia coli DH5α (purchased from Beij...
Embodiment 2
[0054] Example 2 standard curve drawing
[0055] According to GenBank (GenBank registration number: FJ794180) RHDV VP60 capsid protein gene sequence design and screening PCR primers, and confirmed the specificity of each primer, the nucleotide sequences of the primers as SEQ ID No.1 and 2. Primers were synthesized by Shanghai Yingjun Biotechnology Co., Ltd. The length of the amplified fragment of the primer pair is 96bp.
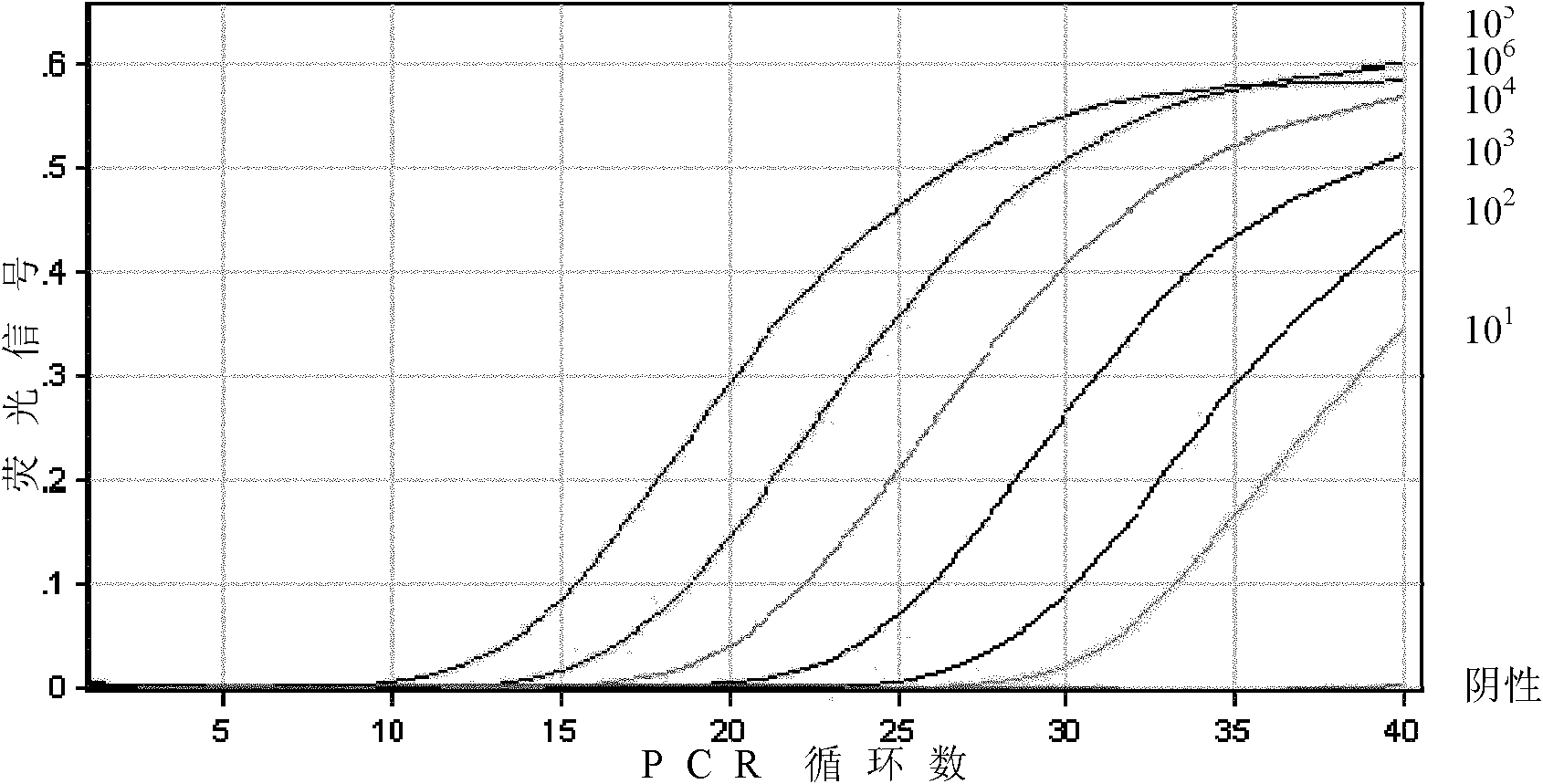
[0056] Using the positive standard obtained in Example 1 as a template, using primers with nucleotide sequences as shown in SEQ ID No.1 and 2 and fluorescent probe VP60p, a one-step RT-PCR method is used for detection. Test results such as image 3 shown. The reaction system in the above RT-PCR is:
[0057] Each 25 μL reaction solution contains: 2×Quant One Step Probe qRT-PCR Master Mix (Quant one-step fluorescent quantitative RT-PCR kit (probe method)) 12.5 μL, 2.5U / μL Hotmaster DNA polymerase 1 μL, 4U / μL Quant reverse transcriptase 0.35 μL, 10 μmol / L ...
Embodiment 3
[0060] Embodiment 3 is to the detection of rabbit liver tissue sample
[0061] 1. Extraction of RNA from rabbit liver tissue samples
[0062] Take 0.2 mL of a 100-fold dilution of RHDV isolates (purchased from Jiangsu Academy of Agricultural Sciences), and inject intramuscularly into 3-month-old unimmunized rabbits. After 3 days, some rabbits died. The liver tissues of 10 dead rabbits were collected, and the rabbit liver tissue samples were extracted using the RNAprep pure animal tissue total RNA extraction kit (purchased from Tiangen Biochemical Technology Co., Ltd.).
[0063] (1) Add 300μL lysate solution RL to 200mg liver tissue, thoroughly grind it into a homogenate, add 590μL RNase-free ddH 2 O and 10 μL proteinase K, mix well and treat at 56°C for 10 min.
[0064] (2) Centrifuge the obtained liquid at 12000 rpm for 5 min, and take the supernatant.
[0065] (3) Slowly add 200 μL of absolute ethanol to the supernatant, mix well, put the obtained solution and the precipi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com