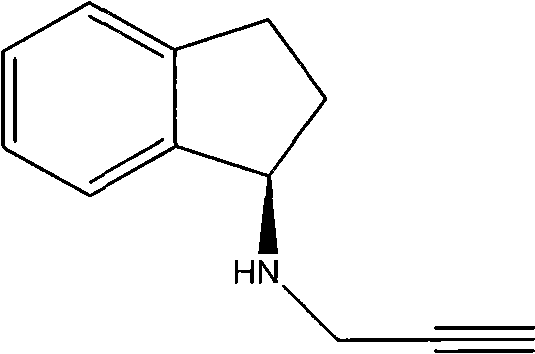
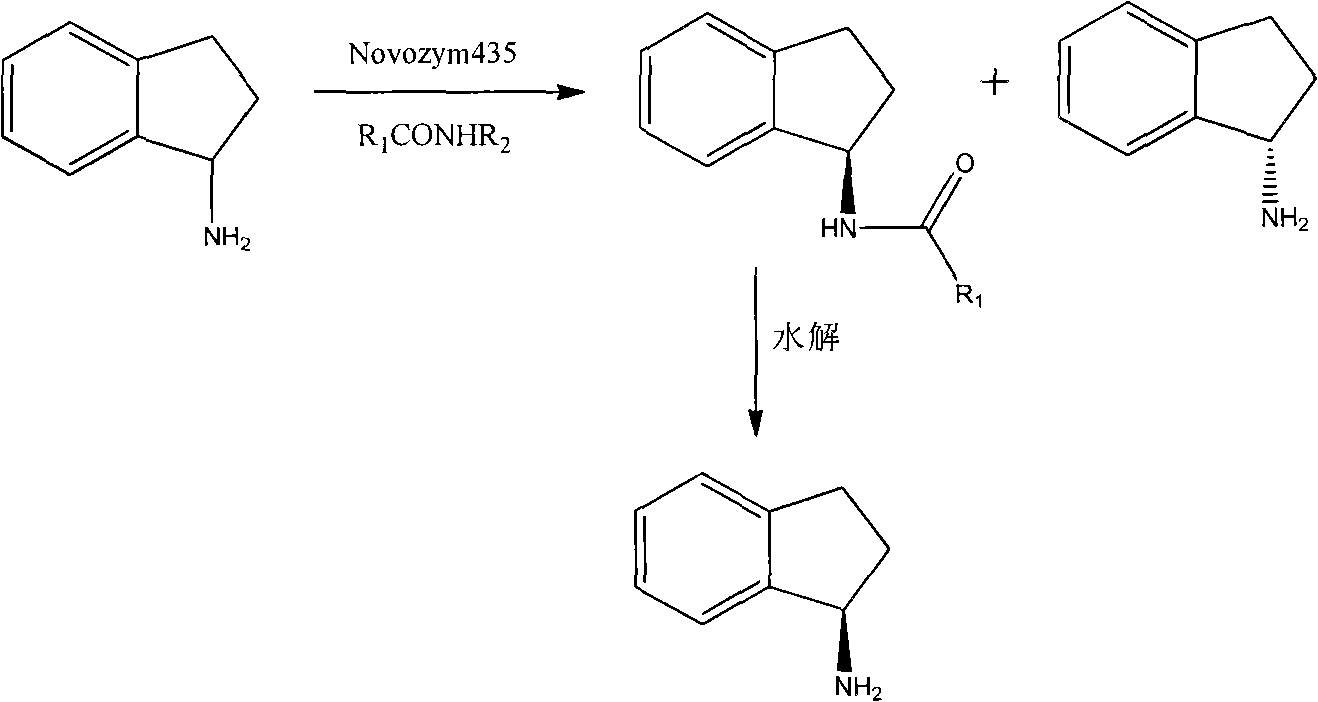
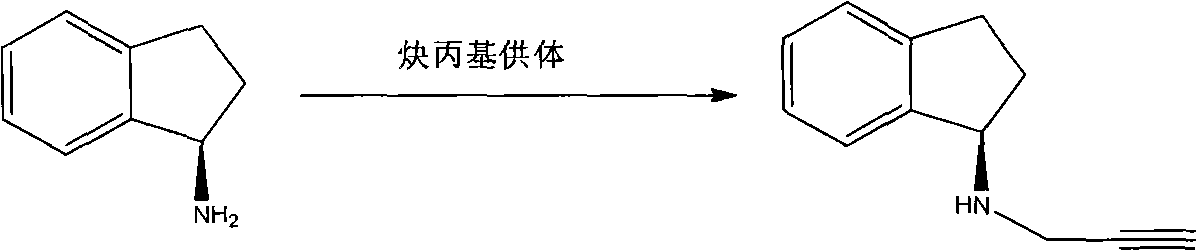
Method for preparing rasagiline
An aminoindan and reaction technology, applied in the field of chemical synthesis, can solve the problems of low optical purity of the product, cumbersome reaction operation, etc., and achieve the effects of reducing the pollution of "three wastes", high reaction yield and easy purchase.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 (R)-1-aminoindan
[0030] Dissolve 13.3g (100mmol) (R,S)-1-aminoindan and 17.6g (200mmol) ethyl acetate in 400ml n-hexane, then add 8g Novozym 435, and stir the reaction at room temperature for 24h. After the reaction, the immobilized enzyme was filtered out, 200 ml of distilled water was added to the filtrate, the pH was adjusted to 5.0 with 1.2 mol / L hydrochloric acid solution, and the liquid was separated. The organic layer was first washed with 100ml of saturated ammonium chloride solution, then washed with saturated sodium chloride solution to neutrality, added anhydrous magnesium sulfate to dry, evaporated to dryness under reduced pressure, and left overnight to obtain a flaky light yellow solid, which was (R )-amide of 1-aminoindane.
[0031] Dissolve the produced amide in 4.0 g of triethanolamine, add 8.0 g of 50% NaOH solution, heat to 120° C., and keep the reaction for 2 h. Cool the reaction compound to room temperature, add 2...
Embodiment 2
[0032] The preparation of embodiment 2 (R)-1-aminoindan
[0033] Dissolve 13.3g (100mmol) (R,S)-1-aminoindan and 8.3g (80mmol) methyl methoxyacetate in 400ml isopropyl ether, then add 7.0g Novozym 435, and stir the reaction at room temperature for 7h. After the reaction, the immobilized enzyme was filtered out, 200 ml of distilled water was added to the filtrate, the pH was adjusted to 5.0 with 1.2 mol / L hydrochloric acid solution, and the liquid was separated. The organic layer was first washed with 100ml of saturated ammonium chloride solution, then washed with saturated sodium chloride solution to neutrality, added anhydrous magnesium sulfate to dry, evaporated to dryness under reduced pressure, and left overnight to obtain a flaky light yellow solid, which was (R )-amide of 1-aminoindane.
[0034] Dissolve the produced amide in 4.0 g of triethanolamine, add 8.0 g of 50% NaOH solution, heat to 120° C., and keep the reaction for 6 h. Cool the reaction compound to room temp...
Embodiment 3
[0035] The preparation of embodiment 3 (R)-N-propargyl-1-aminoindan
[0036] Add 100ml isopropyl ether, 160ml 10% Na in the 500ml three-necked flask 2 CO 3 solution (with Na 2 CO 3 150mmol), at room temperature, add (R)-1-aminoindane 13.3g (100mmol) while stirring, and stir vigorously for 20min. Slowly add 22.5 g of propargyl benzenesulfonate (110 mmol) dropwise to the system, and rinse the dropping funnel with 20 ml of isopropyl ether after the dropwise addition. The temperature was raised to 60° C., and the reaction was kept for 7 hours.
[0037] Pour the reaction solution into a 1L separatory funnel, wash the container with 30ml of isopropyl ether, then pour into the separatory funnel, shake, let stand, and separate layers to obtain an organic phase (upper layer). The aqueous layer was extracted twice with isopropyl ether, 30 ml each time, and the organic phases were combined.
[0038] Add 1.2 mol / L hydrochloric acid to the organic phase and extract three times, respe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com