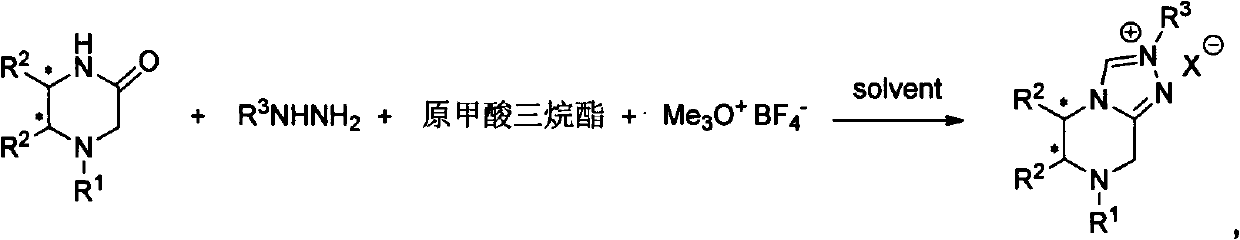Chiral center nitrogen heterocyclic carbine precursor salt with quadrol skeleton, synthetic method and application
A technology of nitrogen heterocyclic carbene and chiral center, applied in the field of chiral nitrogen heterocyclic carbene precursor salt, synthesis, can solve the problem of limited skeleton structure and the like, achieve simple synthesis method, high practical application value, enantioselectivity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
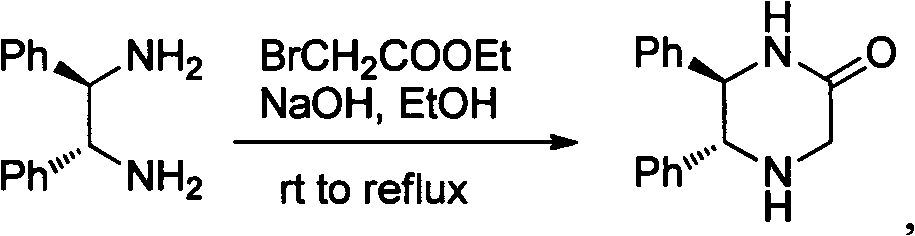
[0024] (1) Synthesis of (5R, 6R)-5,6-diphenylpiperazin-2-one
[0025]
[0026] Among them, rt means room temperature; reflux means reflux.
[0027]Slowly add 1.65g (1.1mL, 10mmol) of ethyl bromoacetate in 20mL of absolute ethanol solution dropwise to (1R, 2R)-1,2-diphenyl-1,2-ethylenediamine (2.12g, 10mmol ) and NaOH 0.4g (10mmol) in 30mL absolute ethanol solution, it takes about three hours. React at room temperature for two days, and reflux at 80 degrees overnight. The solvent was removed under reduced pressure and separated by column chromatography (ethyl acetate / methanol=20 / 1) to obtain 1.4 g (5.55 mmol, yield 54%) of the product as a white solid.
[0028] P1(5R,6R)-5,6-Diphenylpiperazin-2-one
[0029] (and its enantiomers)
[0030] 1 H NMR (300MHz, CDCl 3 )δ7.11-6.86(m, 10H), 6.51(s, 1H), 4.40(d, J=9.0Hz, 2H), 3.63(m, 3H), 2.19(brs, 1H). 13 C NMR (75MHz, CDCl 3 )δ169.7, 138.5, 138.4, 128.26, 128.23, 128.17, 128.09, 127.7, 127.4, 65.8, 64.4, 50.0.
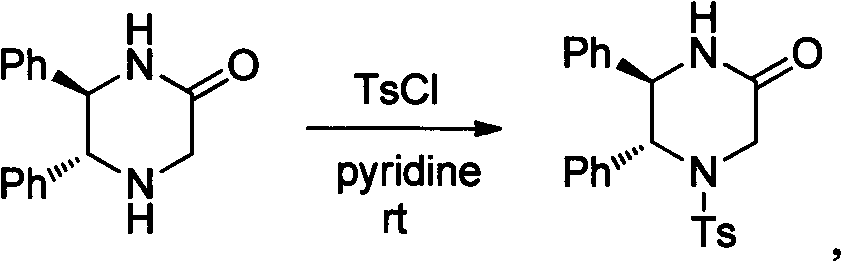
[0031] (2...
Embodiment 2
[0184] Application of Nitrogen Heterocyclic Carbenes in the Synthesis of Chiral Benzopyrone Compounds
[0185]
[0186] Among them, base means base, and solvent means organic solvent.
[0187] General experimental operation: Dissolve the catalyst precursor in xylene, add alkali, and stir at room temperature for 0.5 hours. Add 0.1 mmol of the substrate and react at room temperature. After TLC tracking the reaction is complete, add distilled water at 0°C to quench, extract with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, and purify by column chromatography (petroleum ether: ethyl acetate) to obtain the product, ee value Determined by chiral HPLC.
[0188]
[0189] Colorless oil, 95% yield, 93% ee [chiral column AD-H, n-hexane / isopropanol=97 / 3, v=0.7mL·min -1 , λ=254nm, t(major)=23.1min, t(minor)=34.9min]; [α] D 20 =-6.8 (c=1.0, CH 2 Cl 2 ). 1 H NMR (300MHz, CDCl 3 )δ7.89 (dd, J=1.8, 7.8Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com