Method and kit for detecting gene mutation of human cytochrome P450 CYP2C19
A P450CYP2C19, cytochrome technology, applied in the field of molecular biology, can solve problems such as increased blood drug concentration, failure to reach effective blood drug concentration, and drug side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Detection kit of human cytochrome P450CYP2C19 gene mutation according to the present invention
[0046] The CYP2C19 genotyping kit of the present invention includes four units of PCR amplification reaction, amplification product purification, sequencing reaction, homozygous sequencing product and sample loading.
[0047] PCR Amplification Reaction Unit
[0048] The PCR amplification unit for detecting M1 and M2 contains one tube of reaction reagent and one tube of positive reference substance.
[0049] 1. PCR reaction reagent
[0050] It is prepared by specific primer pair, PCR buffer, dNTP mixture and Taq DNA polymerase according to the optimized reaction concentration.
[0051] 1) Specific primers: 50-100 nmol / L each for the forward and reverse primers.
[0052] Oligo6 software was used to design specific PCR primers for amplifying gene fragments containing M1 or M2 mutation sites, and the sequences were:
[0053] The forward primer (M1-F) for detect...
Embodiment 2
[0100] Embodiment 2: Apply the method and kit of the present invention to carry out CYP2C19 mutation detection equipment:
[0101] PCR amplification instrument, ABI 3130 genetic analyzer, ultra-clean workbench, high-speed desktop centrifuge, water bath, electrophoresis apparatus, etc.
[0102] Extract genomic DNA:
[0103] Sodium citrate or sodium citrate anticoagulant peripheral venous blood 100μl, add 2-5 times the volume of human erythrocyte lysate, mix well, ice bath for 10-15 minutes, centrifuge at 2000rmp for 5 minutes, discard the supernatant to collect lymphocytes, and use whole Blood Genomic DNA Extraction Kit to extract genomic DNA. Various methods can be used to extract whole blood genomic DNA, such as alkaline lysis, silica gel, silica adsorption, magnetic bead adsorption, chromatography column, etc. Currently, there are many commercial kits to choose from, and column layer is recommended Analysis kit.
[0104] PCR reaction
[0105] 1) Take out the PCR reaction...
Embodiment 3
[0138] Example 3: Performing Genotype Analysis
[0139] Based on biological information analysis of sequencing sequences and mutation sites, determine the sequence characteristics of M1 and M2 wild-type and mutant alleles:
[0140] Amplify and analyze according to the method described in Example 1 and Example 2. Taking the detection of M1 mutation as an example, the bioinformatics analysis is described as follows:
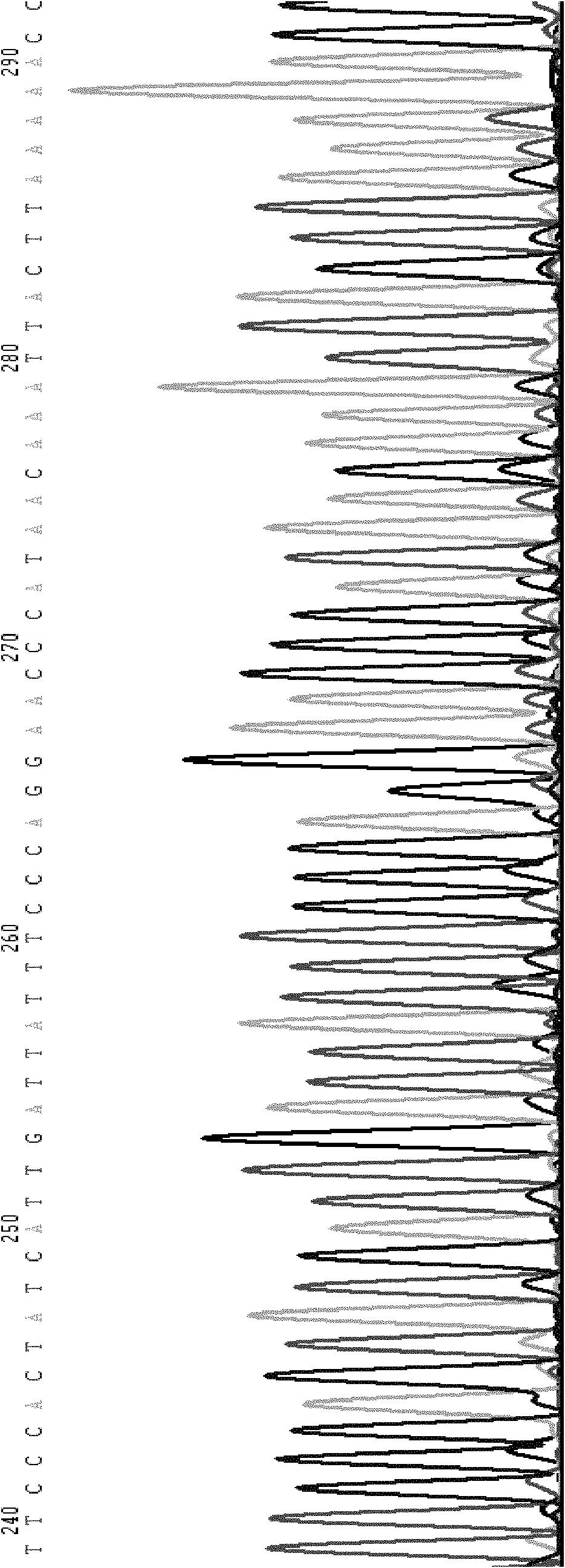
[0141] The expected sequence of the forward sequencing of the amplified product of the detection M1 mutation primer is:
[0142] AAAGCAGGTATAAGTCTAGGAAATGATTATCATCTTTGATTCTCTTGTCAGAATTTTCTTTCTCAAATCTTGTATAATCAGAGAATTACTACACATGTACAATAAAAATTTCCCATCAAGATATACAATATATTTTTTATATTTAGTTTTAAATTACAACCAGAGCTTGGCATATTGTATCTTAACTTTATTATTAAATGCTTTTAATTTTAATAAATTATTGTTTTTCTTAGATTTACTTCG GGAACCCATAACAAATTACTTAAAAACCTTGCTTTTATGGAAAGTGATATTTTGGAGAAAGTAAAAGAACACCAAGAATCGATGGACATCAACAACCCTCGGGACTTTATTGATTGCTTCCTGATCAAAATGGAGAAGGTAAAATGTTAACAAAAGCTTAGTTATGTGACTGCTTGCGTATTTGTG (the nucl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com