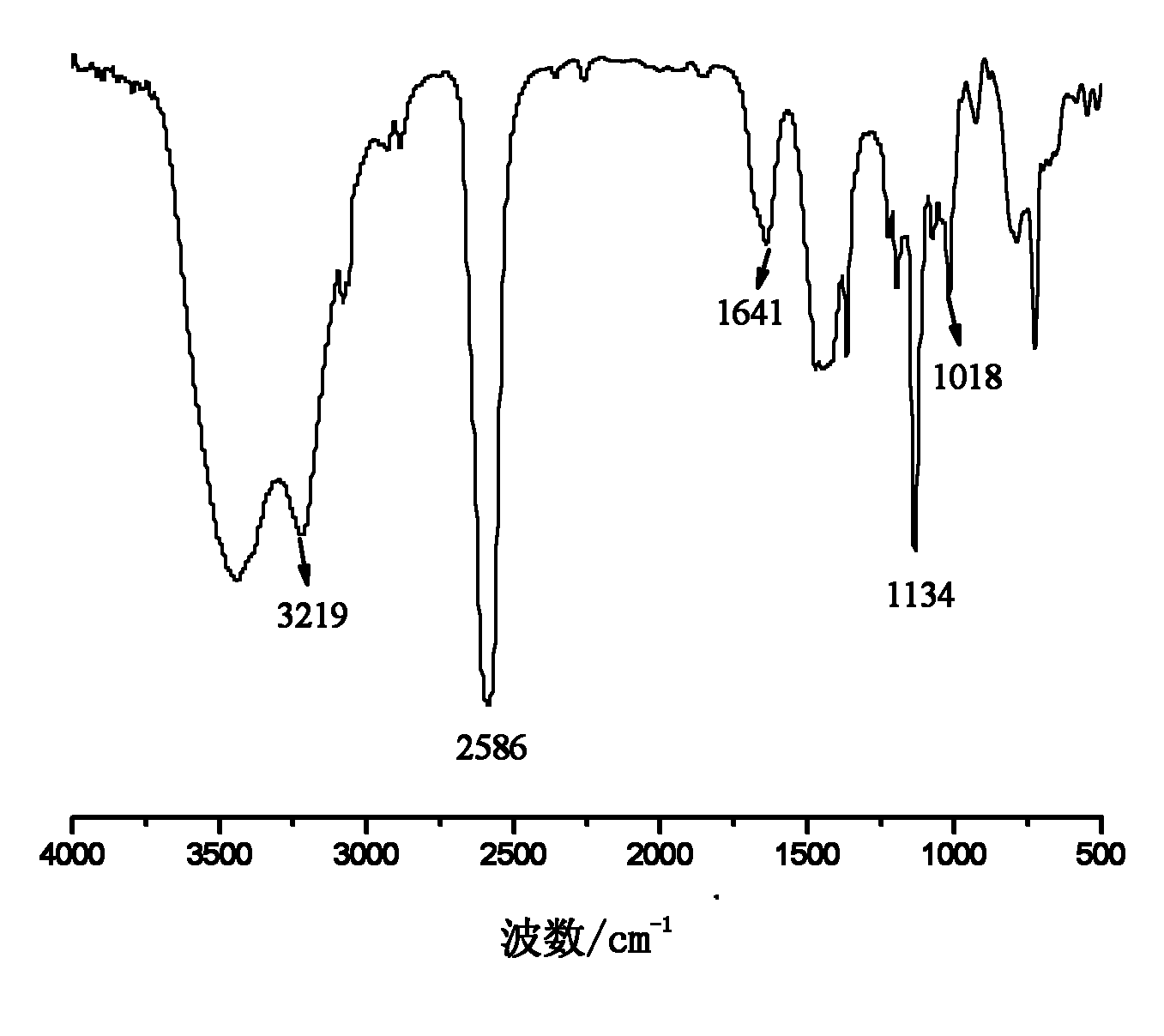
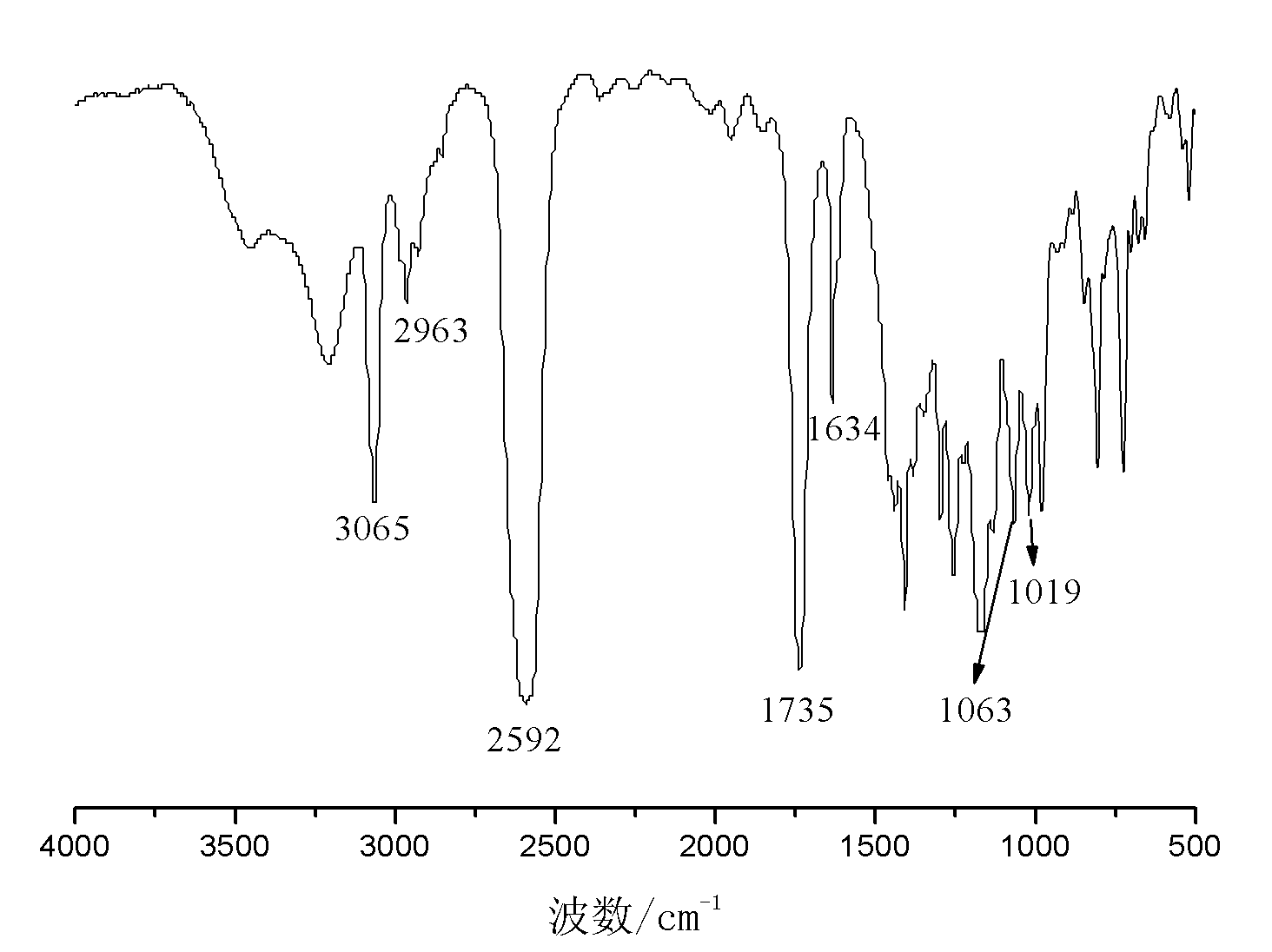
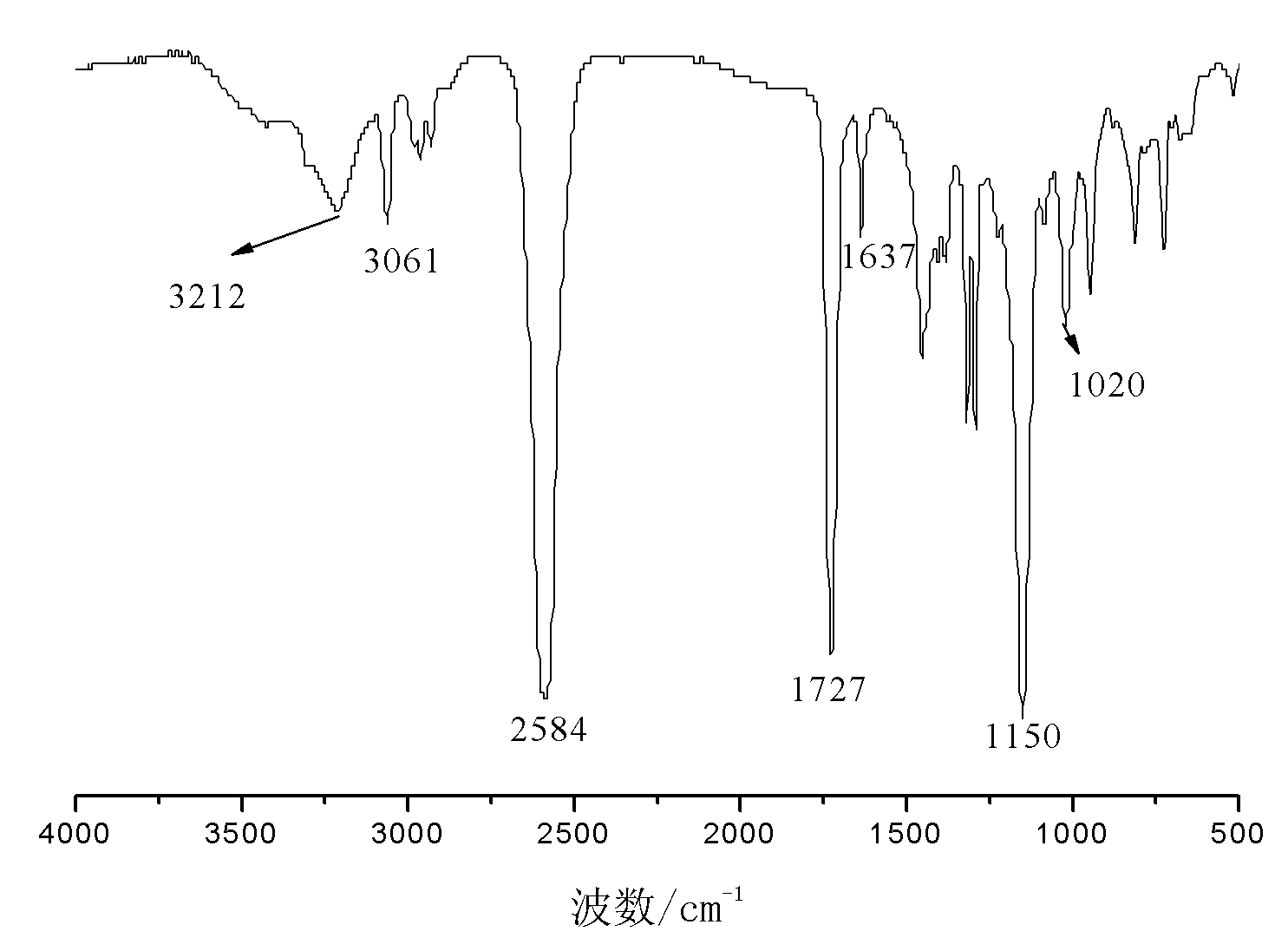
Carborane and preparation method thereof
A carborane and formula technology, applied in the field of carborane, can solve problems such as migration, and achieve the effects of low synthesis cost, strong reactivity and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of allyloxymethylcarborane
[0036] In a three-necked flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, add 200 g of ether, 70.1 g of pyridine and 20.6 g of allyl alcohol, and add 50.8 g of propyne bromide under stirring at room temperature. After the addition, keep warm at 30°C and stir for 12h. After the reaction, the generated salt was removed by filtration, and the solvent was distilled off from the filtrate at 40-45° C. to obtain 25.0 g of light yellow transparent oily liquid allyl propargyl ether with a purity of 99.0% and a yield of 74.2%.
[0037] Add 12.4g of decaborane, 19.2g of allyl propargyl ether and 70g of acetonitrile into a three-necked flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, stir, heat to reflux, and react for 16 hours. After finishing the reaction, the reaction solution was cooled to room temperature, filtered, and the fil...
Embodiment 2
[0040] Embodiment 2: the preparation of allyloxyethoxymethylcarborane
[0041] In a three-necked flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, add 150g of tetrahydrofuran, 35.5g of pyridine and 36.3g of ethylene glycol allyl ether, and add 26.7g of chloropropyne under stirring at room temperature. After the addition, keep warm at 65°C and stir for 18h. After the reaction was finished, the generated salt was removed by filtration, and the filtrate was distilled to remove the solvent at 65-70° C. to obtain 26.8 g of light yellow transparent oily liquid allyloxyethoxypropargyl ether with a purity of 98.9% and a yield of 54.6%.
[0042]Add 6.2g of decaborane, 14.1g of allyloxyethoxypropargyl ether and 125g of acetonitrile into a three-neck flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, stir, heat to reflux, and react for 16h. After finishing the reaction, the reaction solution was coo...
Embodiment 3
[0045] Embodiment 3: the preparation of hexeneoxymethylcarborane
[0046] In a three-necked flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, 280g of tetrahydrofuran, 33.5g of pyridine and 37.6g of 5-enylhexanol were added, and 50.8g of propyne bromide was added under stirring at room temperature. After the addition, keep warm at 65°C and stir for 16h. After the reaction, the generated salt was removed by filtration, and the filtrate was distilled to remove the solvent at 65-70° C. to obtain 28.3 g of light yellow transparent oily liquid allyloxyethoxy propargyl ether with a purity of 97.6% and a yield of 57.7%.
[0047] Add 12.2g of decaborane, 28.0g of allyloxyethoxypropargyl ether and 300g of acetonitrile into a three-necked flask equipped with a reflux condenser, a thermometer and a constant pressure dropping funnel, stir, heat to reflux, and react for 20 hours. After finishing the reaction, the reaction solution was cooled to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com