Chlorambucil/layered double hydroxides nano-hybrid and preparation method thereof
A chlorambucil and nano-hybrid technology, which is applied in the preparation of oxides/hydroxides, pharmaceutical formulations, drug combinations, etc., can solve problems such as serious toxic side effects, nervous system damage, etc., to reduce toxic side effects, reduce Drug toxicity and side effects, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] a. 5.13g (0.02mol) Mg (NO 3 ) 2 ·6H 2 O and 3.75g (0.01mol) Al(NO 3 ) 3 9H 2 O was dissolved in 60 mL of deionized water.
[0033] b. Prepare a 6% ammonia solution.
[0034] c. Dissolve 0.30 g of chlorambucil in 40 mL of ethanol / water solution with a volume ratio of 1:1 containing 0.2 mol / L NaOH to prepare a chlorambucil solution with a concentration of 0.025 mol / L.
[0035]d. Add the solution of step b to the solution of step a, stir and control the pH to 9.5, and the reaction temperature is 30°C; the reaction time is 2 hours, then age the obtained slurry at 30°C for 3 hours, filter, and wash with water to neutral , Peptized at 80°C for 24 hours to obtain layered double hydroxides (LDHs); dry to obtain a powder product.
[0036] e. Disperse 0.4 g of the LDHs powder obtained in step d) into the solution in step c), mix well, and react at 60° C. for 3 days to obtain a chlorambucil / LDHs nanohybrid.
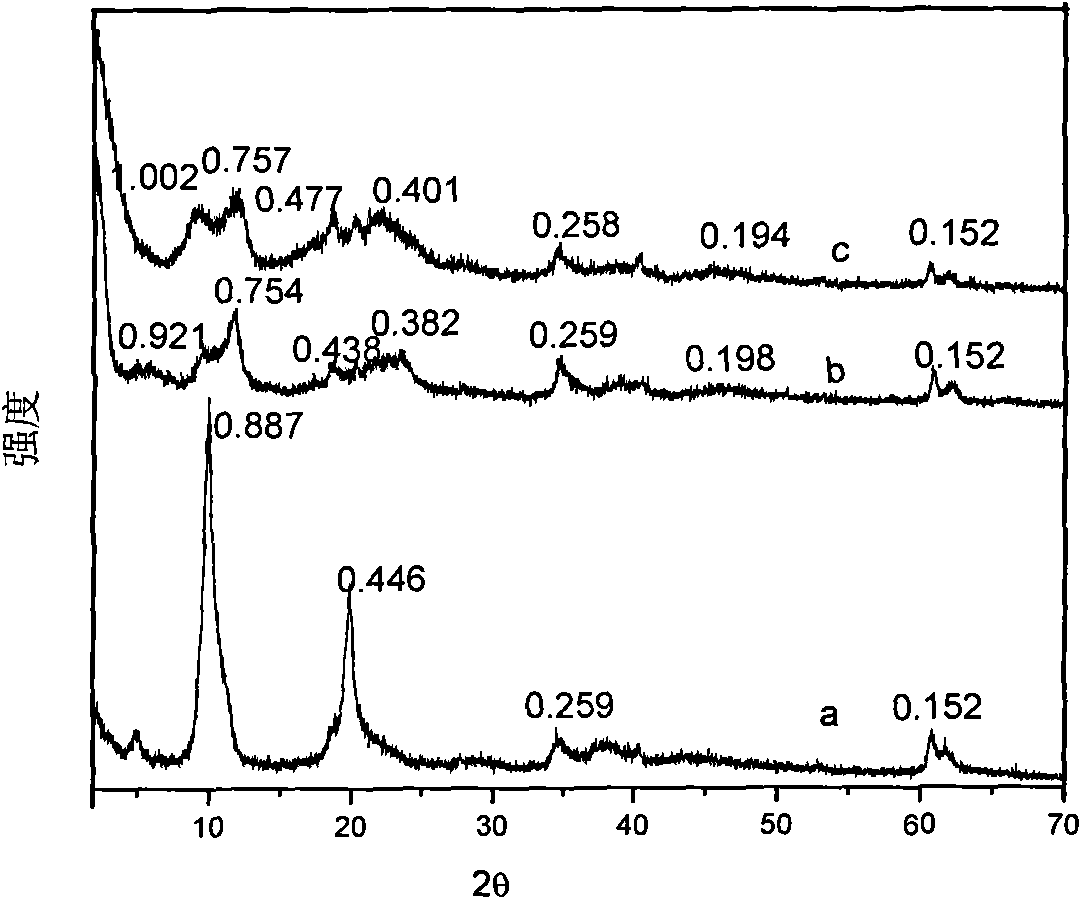
[0037] By XRD spectrum ( figure 1 b) It can be seen that the ch...
Embodiment 2
[0042] a. 5.13g (0.02mol) Mg (NO 3 ) 2 ·6H 2 O and 3.75g (0.01mol) Al(NO 3 ) 3 9H 2 O was dissolved in 60 mL of deionized water.
[0043] b. Prepare a 6% ammonia solution.
[0044] c. Dissolve 0.60 g of chlorambucil in 40 mL of ethanol / water solution with a volume ratio of 1:1 containing 0.2 mol / L NaOH to prepare a chlorambucil solution with a concentration of 0.05 mol / L.
[0045] d. Add the solution of step b to the solution of step a, stir and control the pH to 9.5, and the reaction temperature is 15°C; the reaction time is 2 hours, then the resulting slurry is aged at 15°C for 3 hours, filtered, and washed with water until neutral , Peptized at 80°C for 24 hours to obtain layered double hydroxides (LDHs); dry to obtain a powder product.
[0046] e. Disperse 0.4 g of the LDHs powder obtained in step d) into the solution in step c), mix well, and react at 60° C. for 3 days to obtain a chlorambucil / LDHs nanohybrid.
[0047] By XRD spectrum ( figure 1 c) It can be seen...
Embodiment 3
[0052] a, 4.06g (0.02mol) MgCl 2 ·6H 2 O and 2.41g (0.01mol) AlCl 3 9H 2 O was dissolved in 30 mL deionized water.
[0053] b. Prepare a NaOH solution with a concentration of 2 mol / L.
[0054] c. Dissolve 0.12 g of chlorambucil in 40 mL of ethanol / water solution with a volume ratio of 1:1 containing 0.2 mol / L NaOH to prepare a chlorambucil solution with a concentration of 0.01 mol / L.
[0055] d. Add the solution of step b to the solution of step a, stir and control the pH to 8, the reaction temperature is 45°C; the reaction time is 1 hour, then age the obtained slurry at 45°C for 8 hours, filter, and wash with water until neutral , Peptized at 60°C for 28 hours to obtain layered double hydroxides (LDHs); dry to obtain a powder product.
[0056] e. Disperse 0.4 g of the LDHs powder obtained in step d) into the solution in step c), mix well, and react at 20° C. for 5 days to obtain a chlorambucil / LDHs nanohybrid.
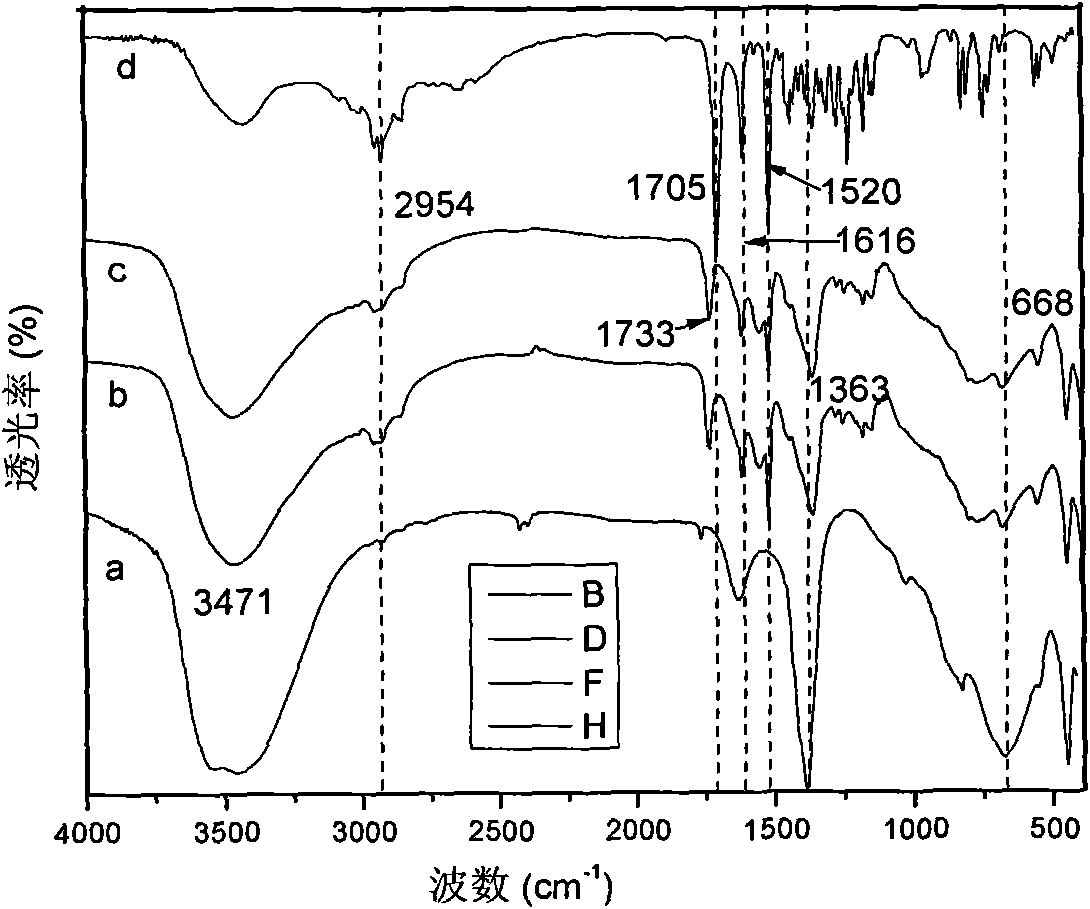
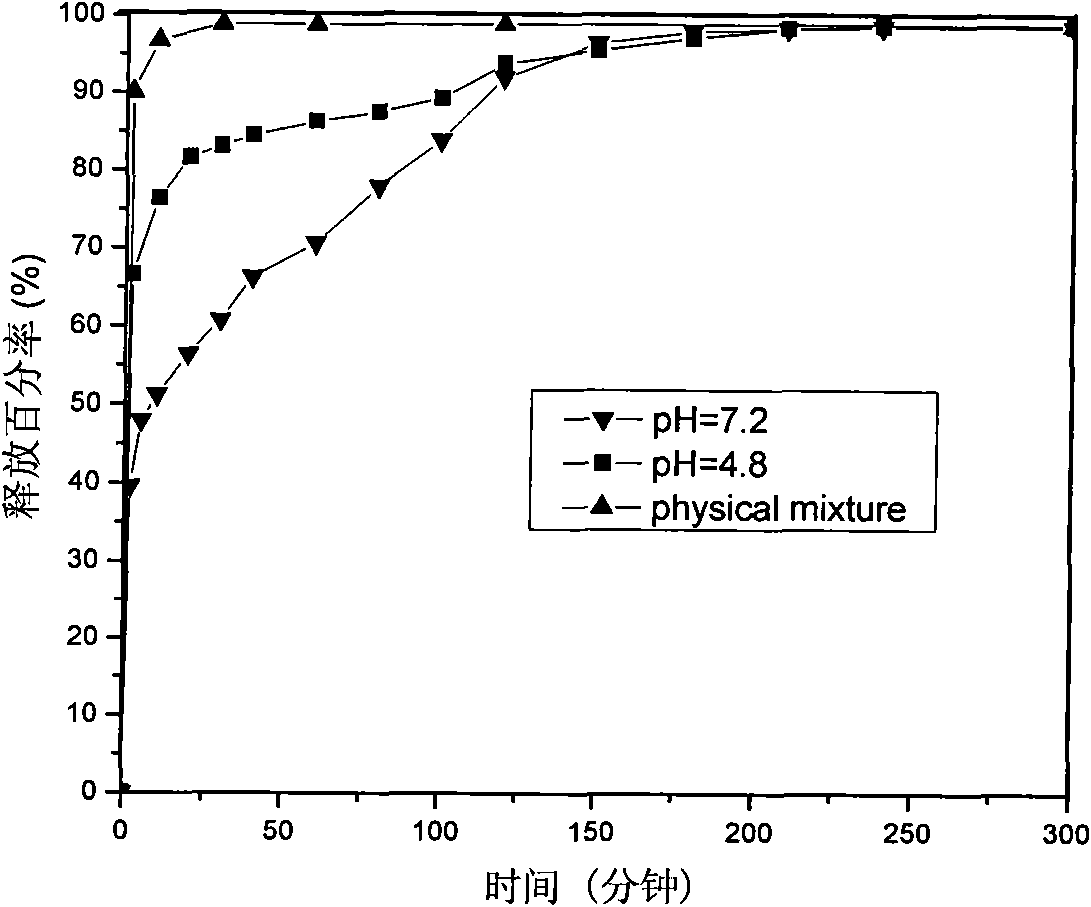
[0057] The sample was analyzed by ultraviolet spectrophoto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com