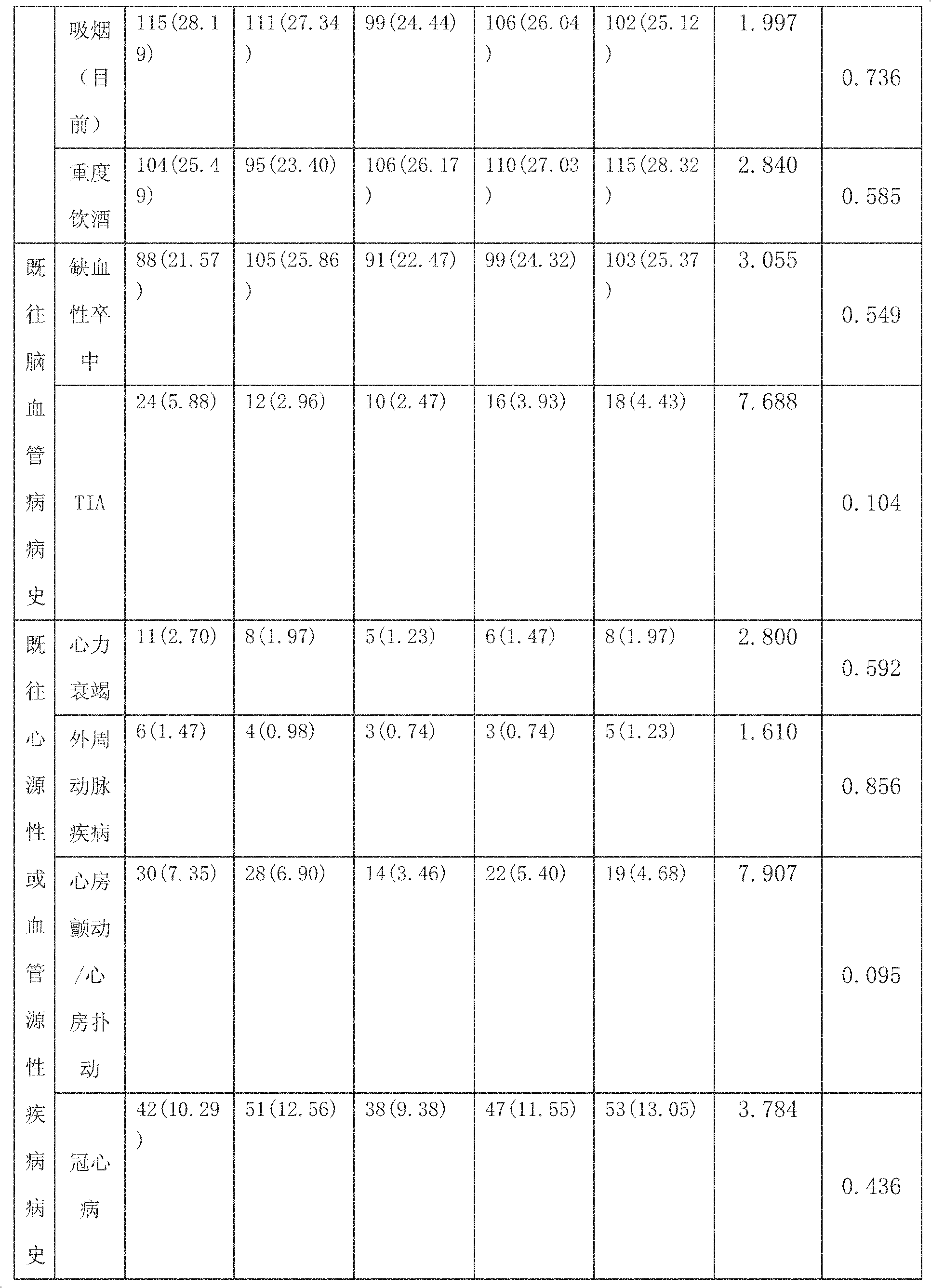Medicinal composition containing clopidogrel and aspirin and application thereof to preparation of medicaments for treating acute non-disabling cerebrovascular diseases
A technology of aspirin and clopidogrel, applied in drug combinations, medical preparations containing active ingredients, cardiovascular system diseases, etc., can solve problems such as unsatisfactory effects, inconvenience, and heavy burden on patients, and achieve simple and convenient compliance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Compound matrix sustained-release tablet of embodiment 1 aspirin and clopidogrel bisulfate
[0024] Aspirin 75mg
[0025] Clopidogrel 75mg
[0026] Hypromellose 150mg
[0027] Lactose 50mg
[0028]Magnesium stearate 3~6mg
[0029] Proper amount of hypromellose ethanol solution
[0030] Preparation process: crush aspirin, clopidogrel, and hydroxypropylmethylcellulose, a matrix sustained-release material, and sieve. Weigh the prescribed amount of aspirin, clopidogrel hydrogen sulfate and mix them with hypromellose and lactose respectively, then add the ethanol solution of hypromellose respectively, mix to make soft material, and granulate with 24-30 nylon sieve. Ventilate and dry at a temperature of 40°C to 50°C for 1.5 to 2.5 hours, granulate, then mix aspirin sustained-release granules and clopidogrel bisulfate sustained-release granules, add magnesium stearate, and compress into tablets to obtain.
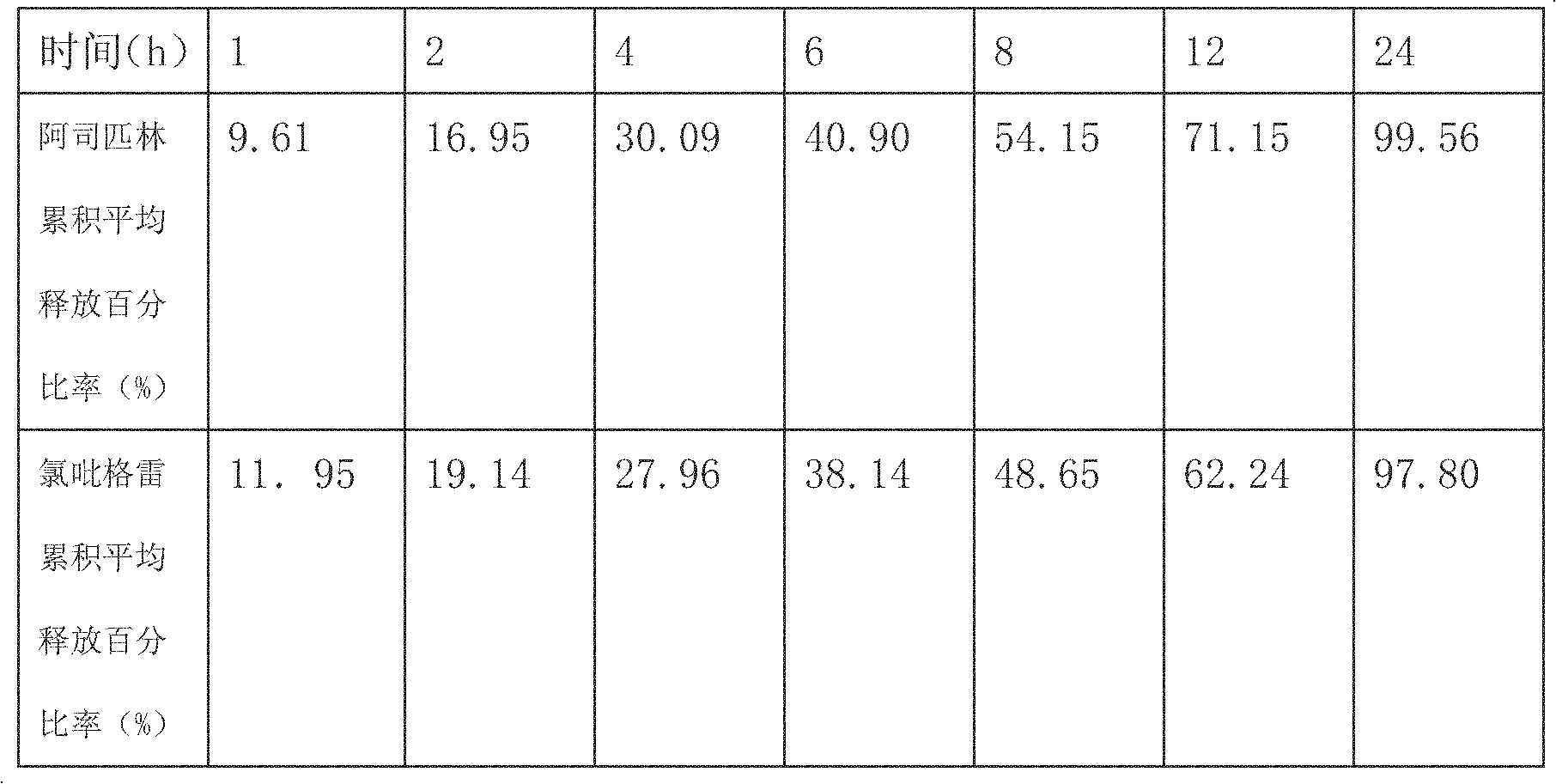
[0031] Determination of the release rate of sustained-release ta...
Embodiment 2
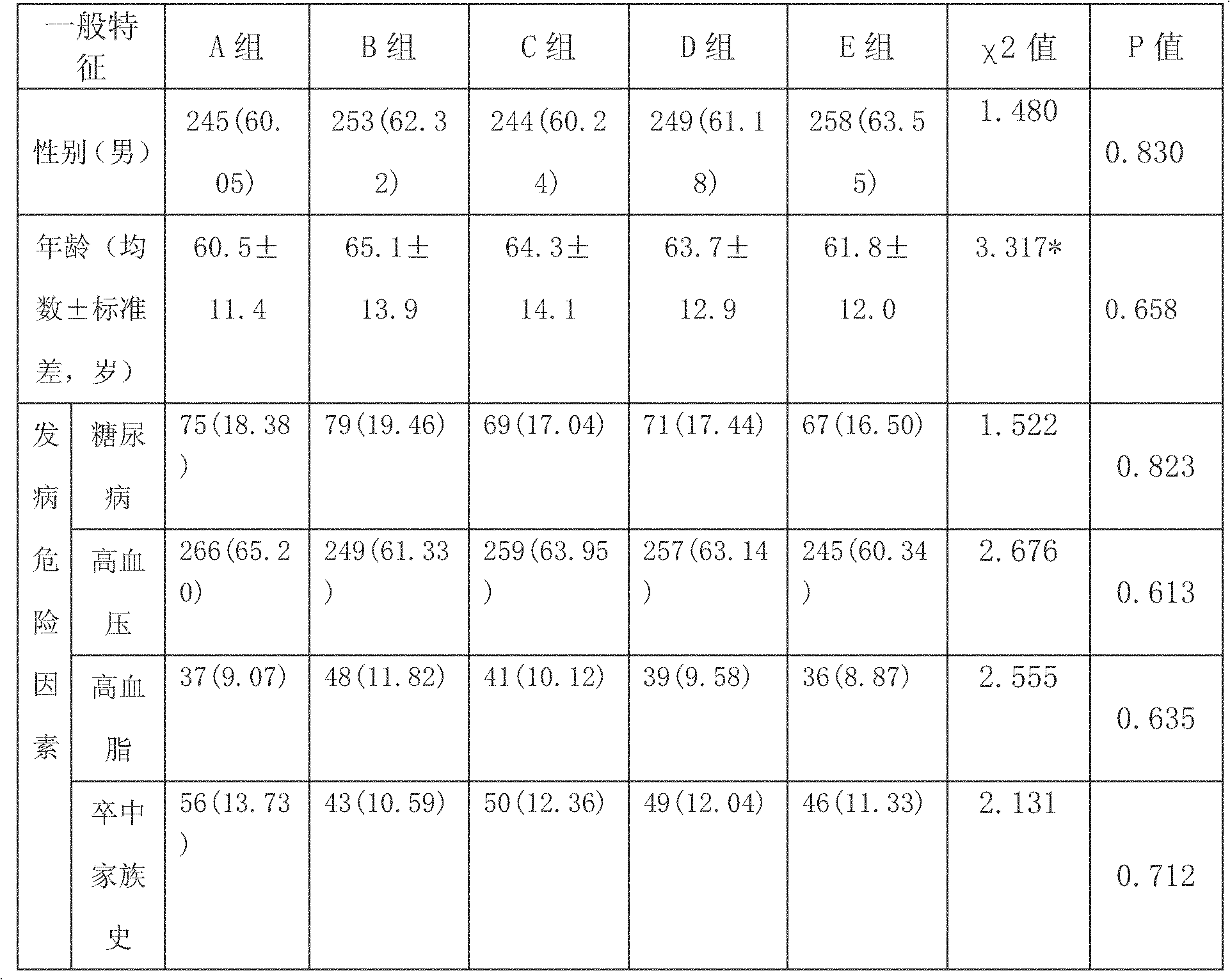
[0034] Example 2 Observational study of combined application of antiplatelet drugs in patients with TIA and minor ischemic stroke
[0035] (1) A randomized double-blind study design is adopted:
[0036] 1. Inclusion criteria for observational studies of combined use of antiplatelet drugs in patients with TIA and minor ischemic stroke:
[0037] 1) Adults, male or female, aged ≥ 40 years;
[0038] 2) Non-disabling ischemic stroke (NIHSS ≤ 3 points on admission), and the study drug can be used within 24 hours of symptom onset. The time to symptom onset was defined as "the time when it last appeared normal".
[0039] 3) Those with medium-to-high risk of stroke (ABCD at admission 2 Patients with TIA (focal cerebral or retinal ischemia-induced neurological dysfunction, which completely disappears within 24 hours) with a score ≥ 4), and the study drug can be used within 24 hours of symptom onset. The time to symptom onset was defined as "the time when it last appeared normal".
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com