Immunological detecting kit and preparation method and using method thereof
An immunological detection and kit technology, which is applied in the field of detection kits for lipoprotein-associated phospholipase A2 protein in human plasma, can solve the problems of increasing detection sensitivity, reducing accuracy, and many steps, so as to improve accuracy and accuracy Improvement, False Positive and False Negative Reduction Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] In the preparation method of the kit of the present invention, the directly detectable label is preferably a quantum dot label or a gold nanometer label. In a preferred embodiment, the method for preparing the antibody is a method for preparing monoclonal antibodies using hybridoma technology. In a further preferred embodiment, it also includes the step of preparing another monoclonal antibody that recognizes an antigenic determinant different from the epitope recognized by the monoclonal antibody. This monoclonal antibody can also be prepared by hybridoma technology, for example, by fermentation Hybridomas were generated by tank culture or intraperitoneal injection in mice.
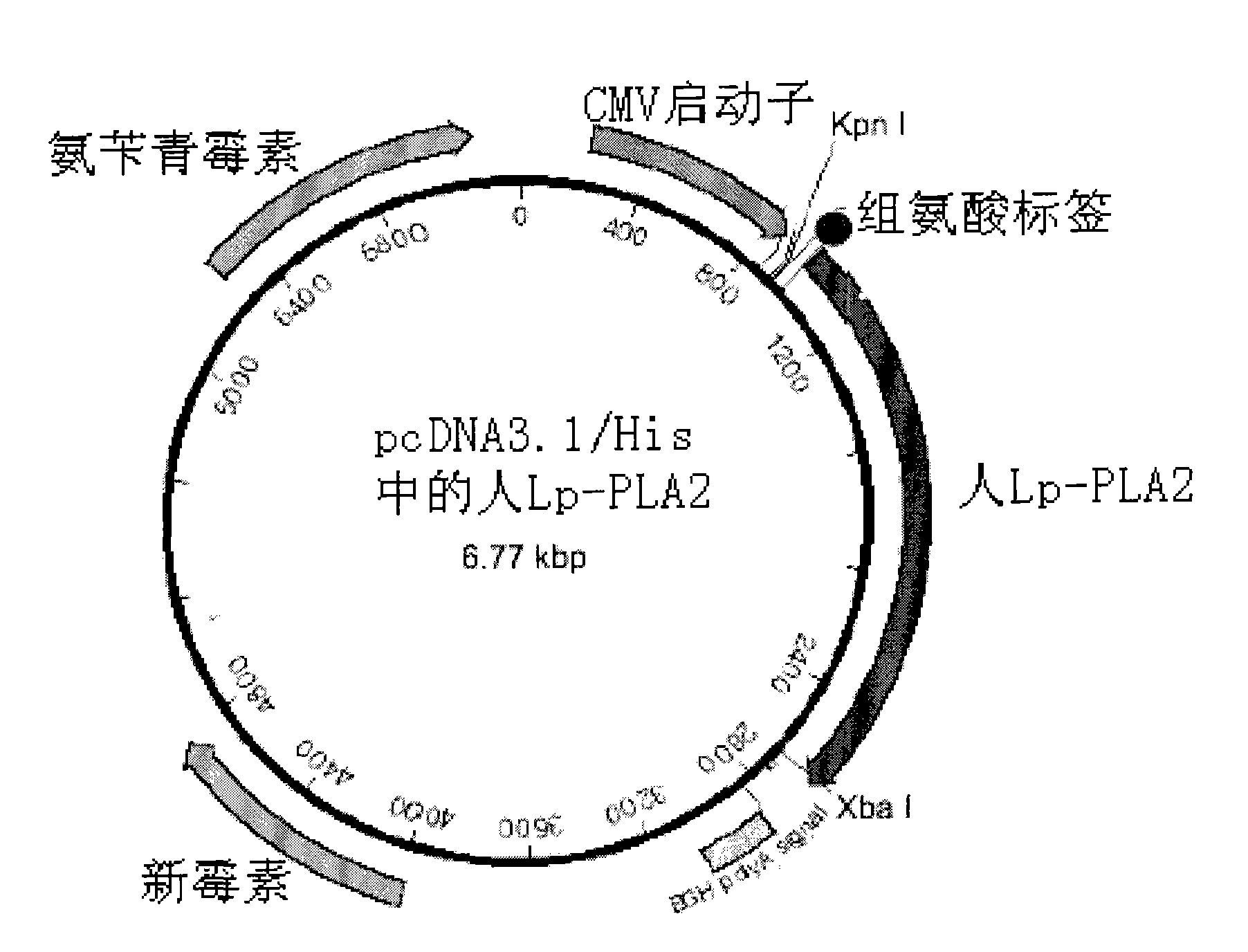
[0033] The kit of the present invention may also include the step of preparing recombinant Lp-PLA2 protein as a standard by fermentation. This standard can be used as a positive control or to create a standard curve.
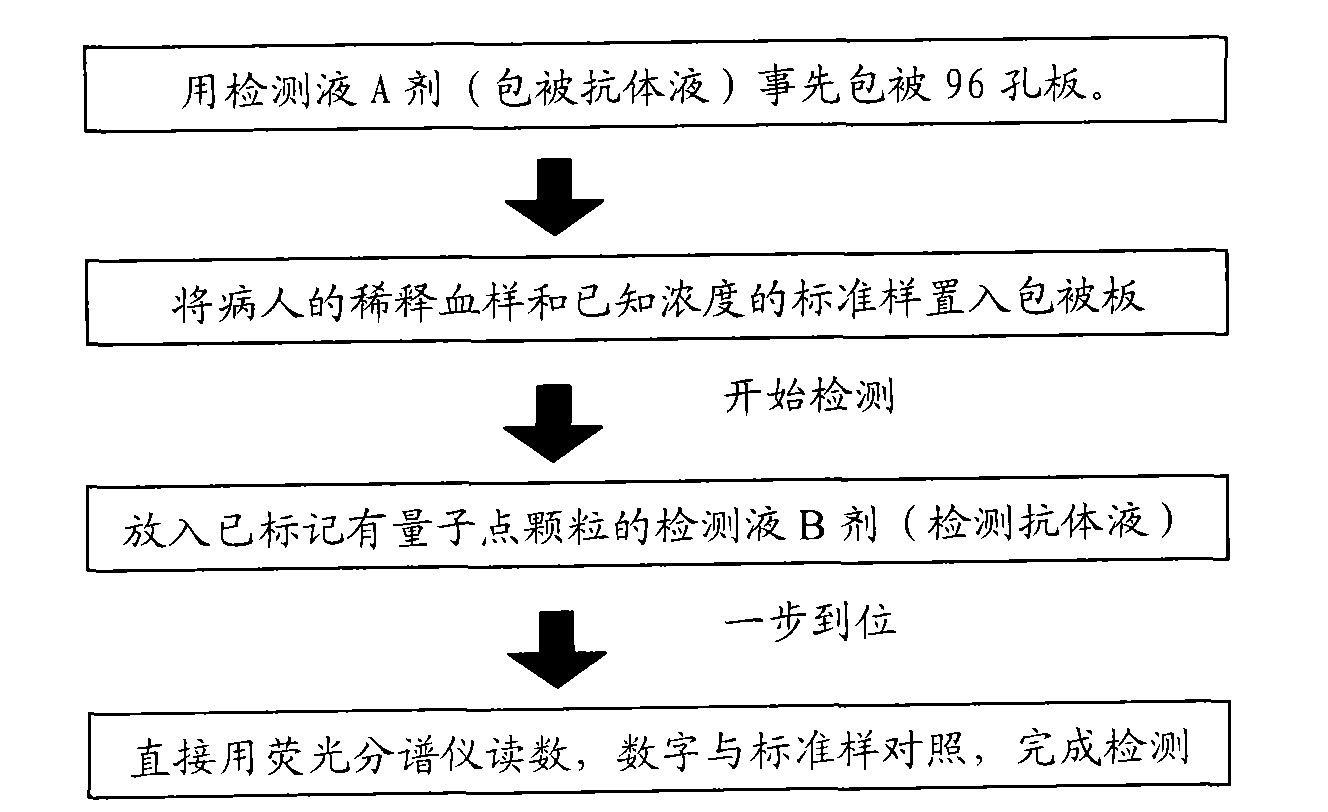
[0034] According to the components of the kit, the method of using the kit of...
Embodiment 1
[0038] 1. Preparation of monoclonal antibody
[0039] 1) PANWNSPLRPGEKYC; 2) SFGQTKIPRGNGPYC; 3) PSQDNDRLDTLWIPC; 4) CDHGKPVKNALDLKF; 5) QHIMLQNSSGIEKYN immunized mice to prepare monoclonal antibody hybridomas, and selected two hybridomas for fermentation The monoclonal antibodies A and B of Lp-PLA2 protein were produced by tank culture or intraperitoneal injection of mice. The specific operation can be found in (Kohler et al., Nature 256:495, 1975; Kohler et al., Eur.J.Immunol.6 :511,1976; Kohler et al., Eur.J.Immunol.6:292,1976; Hammerling et al., InMonoclonal Antibodies and T Cell Hybridomas, Elsevier, N.Y., 1981)
[0040] Purify and concentrate monoclonal antibodies A and B, and carry out formulation, concentration adjustment and sterilized subpackaging respectively. After the monoclonal antibody A is sterilized and subpackaged, it is the detection solution A agent.
[0041] Take monoclonal antibody B and mark it with quantum dots. The following are two methods that can...
Embodiment 2
[0051] The detection of the same process as Example 1, except that the protein to be tested is fibrinogen, or C-reactive protein (C-Reactive Protein, CRP), or thrombosis precursor protein (Thrombus Precursor Protein, TpP), or creatine kinase Creatine Kinase-MB (CK-MB) and other human serum proteins or all other proteins in the detection of cardiovascular and cerebrovascular diseases.
[0052] result
[0053] The detection box reagent of Example 1 was selected to detect the Lp-PLA2 protein concentration of 28 patients diagnosed with coronary heart disease and 21 normal control groups of the same age, and simultaneously compared with the results obtained by using the ELISA method. control. (see Figure 5A and Figure 5B )
[0054] The results showed that two of the 28 cases of the disease group showed false negatives, and one of the 21 normal control groups showed false positives when detected by the ELISA method. However, in the detection results of the kit of the present ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com