Method for synthesizing star-shaped solution polymerized butadiene-styrene rubber by using modified initiator
A technology of solution polymerization of styrene-butadiene rubber and initiator, applied in the field of rubber, can solve the problems of high rubber Mooney viscosity, high price of raw materials, and lack of compatibility of carbon black, so as to save production costs, improve production efficiency, and shorten polymerization time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Under nitrogen protection, add 80ml of cyclohexane, 25mmol of 1,1-diphenylethylene, and 25mmol of n-butyllithium into a purified 250ml polymerization bottle with electromagnetic stirring, and react at 40°C for 80 hours to obtain Modified initiator I-1 shown in structural formula I.
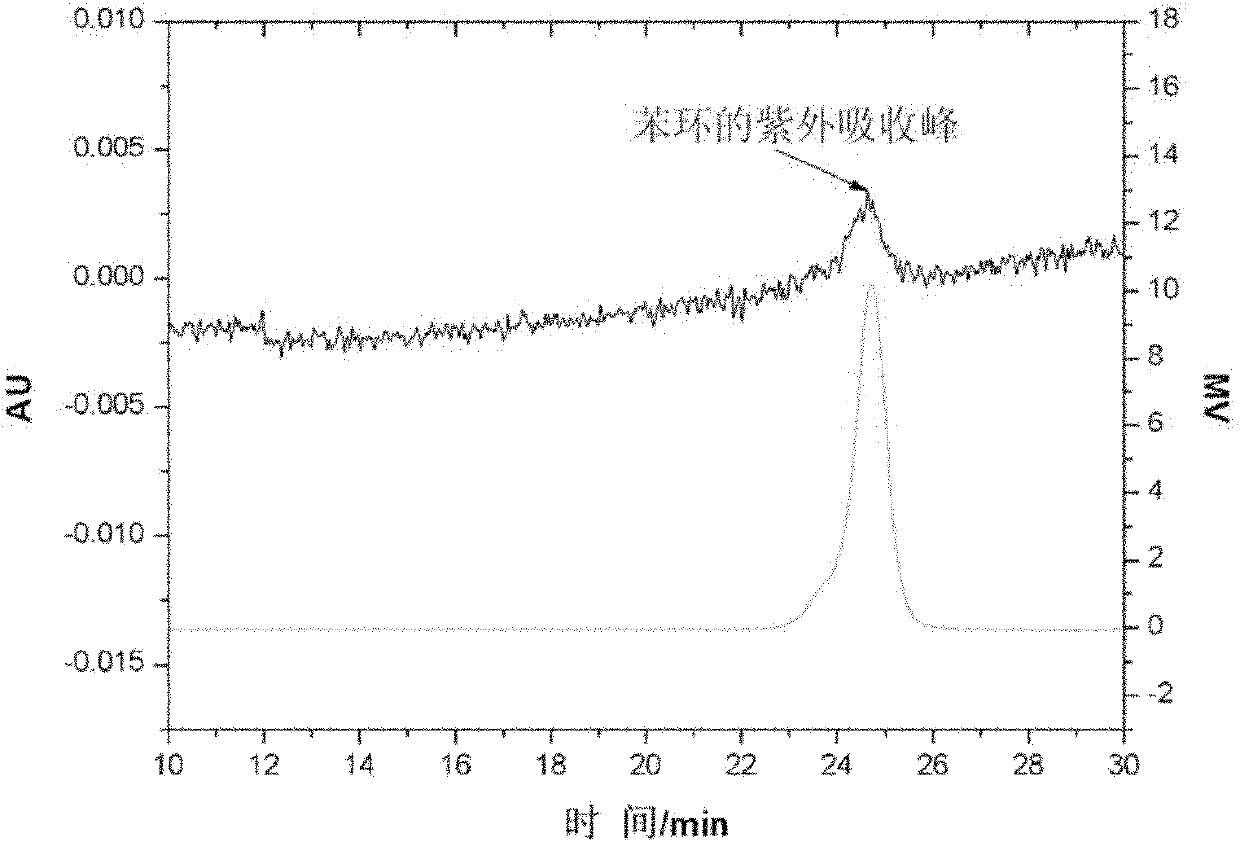
[0043] Under nitrogen protection, add 77ml of cyclohexane, 7.8g of butadiene, and 0.96ml of tetrahydrofuran into a purified 250ml polymerization bottle, take 0.39mmol of the modified initiator I-1 synthesized above, and react at 55°C for 3 hours The reaction was terminated with methanol, and the product was dissolved in cyclohexane, then flocculated with ethanol, then ultrasonically cleaned three times with ethanol, and vacuum-extracted and dried. Linkage with gel permeation chromatography (GPC)-ultraviolet detector, record the number average molecular weight of product polybutadiene to be 22000, as figure 1 . pass figure 1 It can be seen that the characteristic absorption peak of the be...
Embodiment 2
[0045] Under the protection of nitrogen, add 70ml of cyclohexane, 20.2ml of tetrahydrofuran, 25mmol of 1,1-diphenylethylene, and 25mmol of n-butyllithium into a purified 250ml polymerization bottle with electromagnetic stirring, and react at 55°C for 40 Hours, the compound I-2 shown in structural formula I was obtained.
[0046] Under the protection of nitrogen, add 80ml of cyclohexane, 6.4g of butadiene, 1.6g of styrene, and 0.42ml of tetrahydrofuran into a purified 250ml polymerization bottle, and take 0.13mmol of the modified initiator I-2 synthesized above, at 55°C After 3 hours of reaction, the reaction was terminated with methanol, and the product was vacuum-extracted, and the number-average molecular weight of the product measured by GPC and nuclear magnetic resonance was 67,000, the styrene content was 19.8%, and the vinyl content was 49.5%.
Embodiment 3
[0048] Under nitrogen protection, add 100ml of cyclohexane, 30mmol of 1,1-diphenylethylene, and 30mmol of sec-butyllithium into a purified 250ml polymerization bottle with electromagnetic stirring, and react at 40°C for 70 hours to obtain Compound I-3 shown in structural formula I.
[0049] Under nitrogen protection, add 120ml of cyclohexane, 9.0g of butadiene, 3.0g of styrene, and 0.81ml of tetrahydrofuran into a purified 250ml polymerization bottle, and take 0.2mmol of the modified initiator I-3 synthesized above, at 55°C After reacting for half an hour, raise the temperature to 60°C for 1 hour, add SnCl 4 0.056 millimoles were used for the post-coupling reaction. After 2 hours of reaction, the reaction was terminated with methanol solution to obtain the product. The number-average molecular weight was 125,000, the styrene content was 24.6%, and the vinyl content was 46.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com