Catalyst system and process for olefin polymerization
A technology of catalysts and systems, applied in the field of new catalyst systems, which can solve the problems of not giving specific examples or suggestions of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
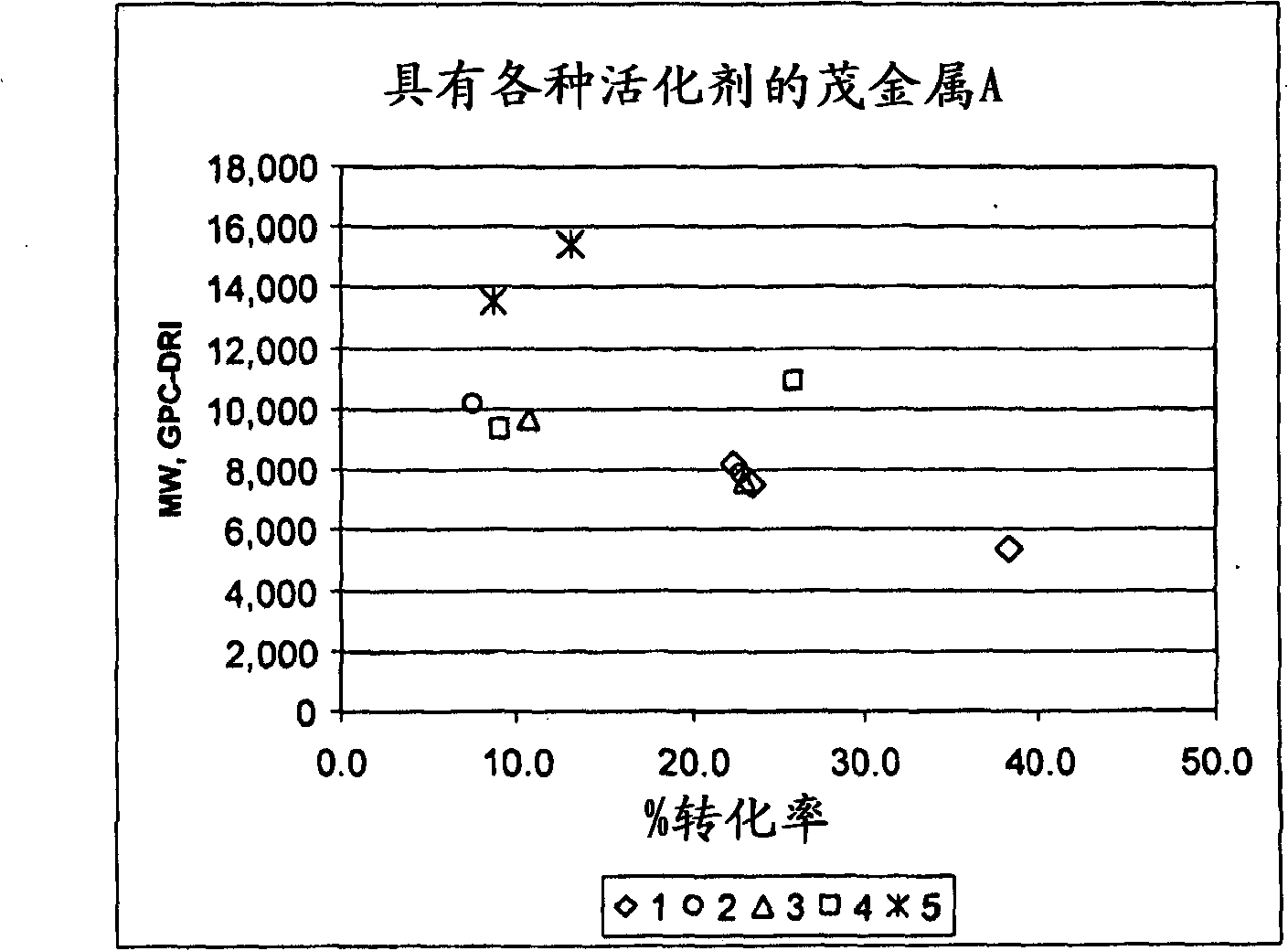
[0260] Polymerization Example 1 (see figure 1 )-batch polymerization of propylene
[0261] at room temperature, in N 2 Under purge, TIBAL (triisobutylaluminum, Aldrich) (0.4 mL, 1.0 M in hexane) was added to the 1 L autoclave, followed by 500 mL of isohexane. Propylene was added in 10 mL increments according to the volume measured by the sight glass. The reaction was heated to temperature, allowed to stabilize and the total reaction pressure was recorded. Catalyst system as a preactivated solution of metallocene and activator in toluene at high pressure N 2 Feed through the catalyst tube while flushing. Rinse and repeat twice. After the polymerization was complete, the reactor contents were cooled to room temperature and the excess pressure was safely vented. Transfer the contents to a glass container and pass the volatiles through N 2 Purify and remove. The polymer was dried in a vacuum oven at 70°C for 2 to 3 hours.
[0262] The metallocenes and activators used i...
Embodiment 2
[0293] Polymerization Example 2 (see Figure 2a , 2b) - continuous polymerization of propylene
[0294] General Polymerization Procedure in Continuous Stirred Tank Reactor: All polymerizations were carried out in a liquid filled single stage continuous reactor system. The reactor was a 0.5-liter stainless steel reactor reactor and was equipped with a stirrer, water cooling / steam heating with thermostat and pressure controller. Solvent, propylene and comonomer were first purified by passing through a three-column purification system. The purification system consisted of an Oxiclear column (model #RGP-R1-500 from Labclear) followed by 5A and 3A molecular sieve columns. Regenerate the purification column periodically whenever there is evidence of low polymerization activity. The 3A and 5A molecular sieve columns were internally regenerated under nitrogen at a setting of 260°C and 315°C, respectively. Molecular sieve material was purchased from Aldrich. Oxiclear columns are...
Embodiment 3
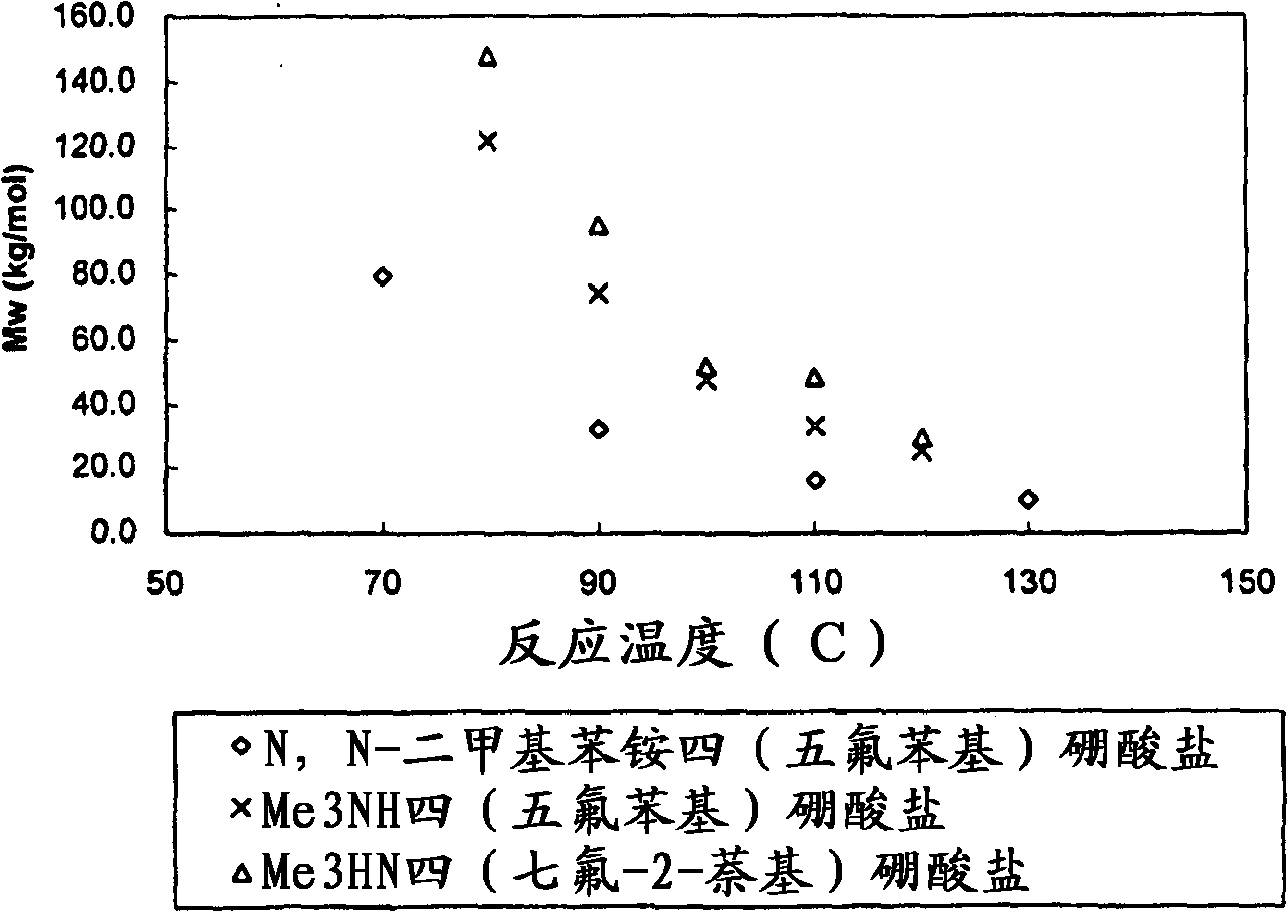
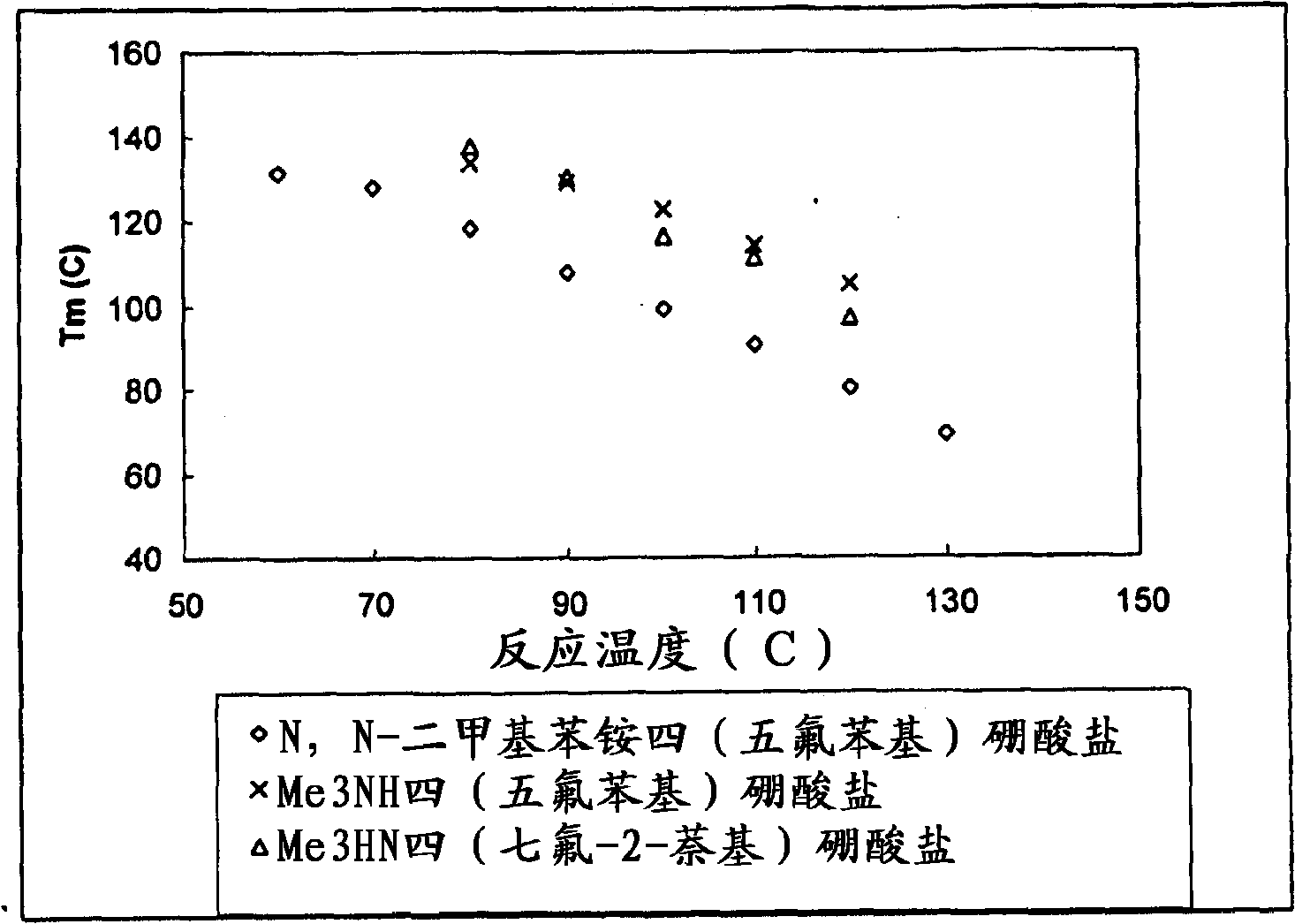
[0310] Polymerization Example 3 (see Figure 3a , 3b) - continuous polymerization of propylene
[0311] Example 3 illustrates the polymerization of propylene using rac-dimethylsilylbis(indenyl)zirconium dimethyl, see Figure 3a and 3b . Using the metallocene with Me 3 NH tetrakis(heptafluoro-2-naphthyl)borate was preactivated in toluene at a molar ratio of about 1:1. The same general polymerization procedure as described above in Example 2 was employed, and the detailed polymerization conditions in these polymerization examples and some analytical data for the material produced are listed in Table 5a. For all polymerization runs, the isohexane feed rate was 80 ml / min, the propylene feed rate was 14 g / min and the metallocene feed rate was 7.835E-07 moles / min. As a comparative example, Table 5b lists the use of rac-dimethylsilane preactivated with dimethylanilinium tetrakis(heptafluoronaphthyl)borate in toluene at a molar ratio of about 1:1. Polymerization conditions and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com