Preparation method of phase change monomer with double-bond end groups
A terminal group and phase change technology, applied in chemical instruments and methods, heat exchange materials, etc., to achieve the effects of wide application range, good high temperature resistance, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In an ice-water bath, dissolve 10 g of polyethylene glycol with a molecular weight of 4000 in 20 ml of dichloromethane, add 0.3 g of triethylamine, and slowly add 0.2 g of acryloyl chloride dropwise; React for 8 hours; put the reaction solution in an ice-water bath, add 150ml of ether, let it stand for 10-15 minutes, and filter under reduced pressure to obtain the phase-change monomer polyethylene glycol acrylate with double bond end groups.
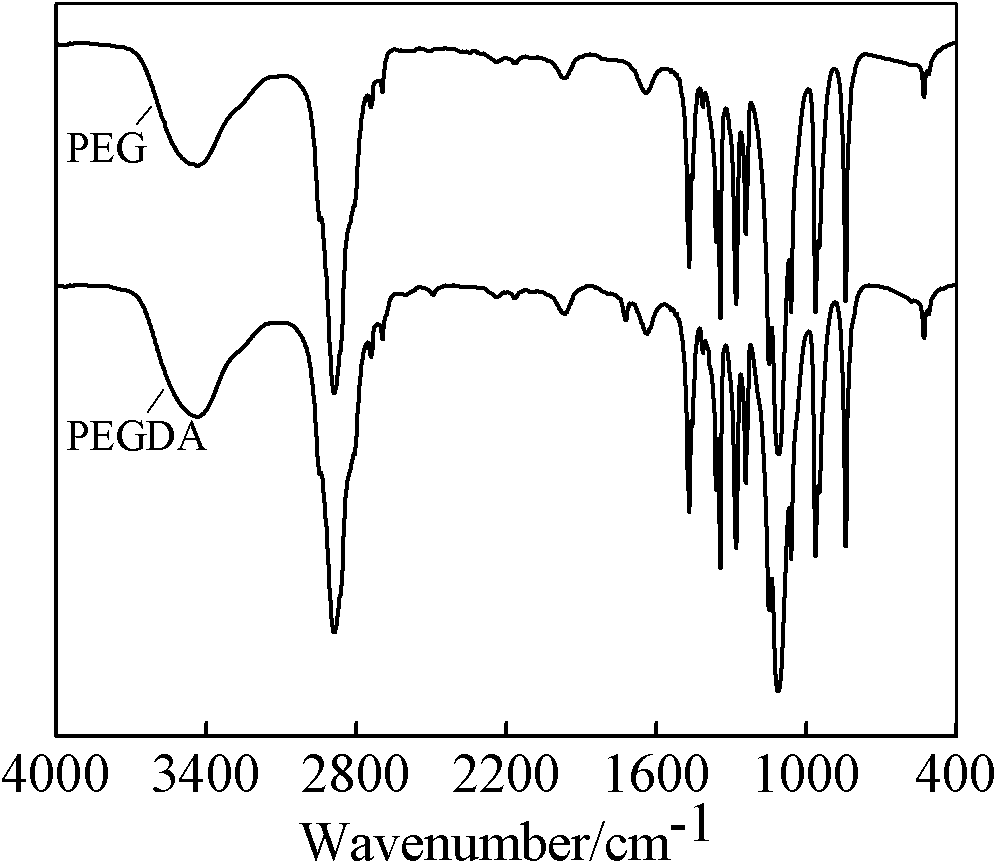
[0034] Carry out infrared spectrum research to above-mentioned phase change monomer (see figure 1 ) analysis, the phase-change monomer prepared by the invention strengthens the characteristic absorption peak of C-O at 1105cm-1, and the absorption peaks of C=O group and C=C double bond appear at 1720cm-1 and 1650cm-1 respectively, indicating that PEG and The acid chloride reacts to form polyethylene glycol acrylate.
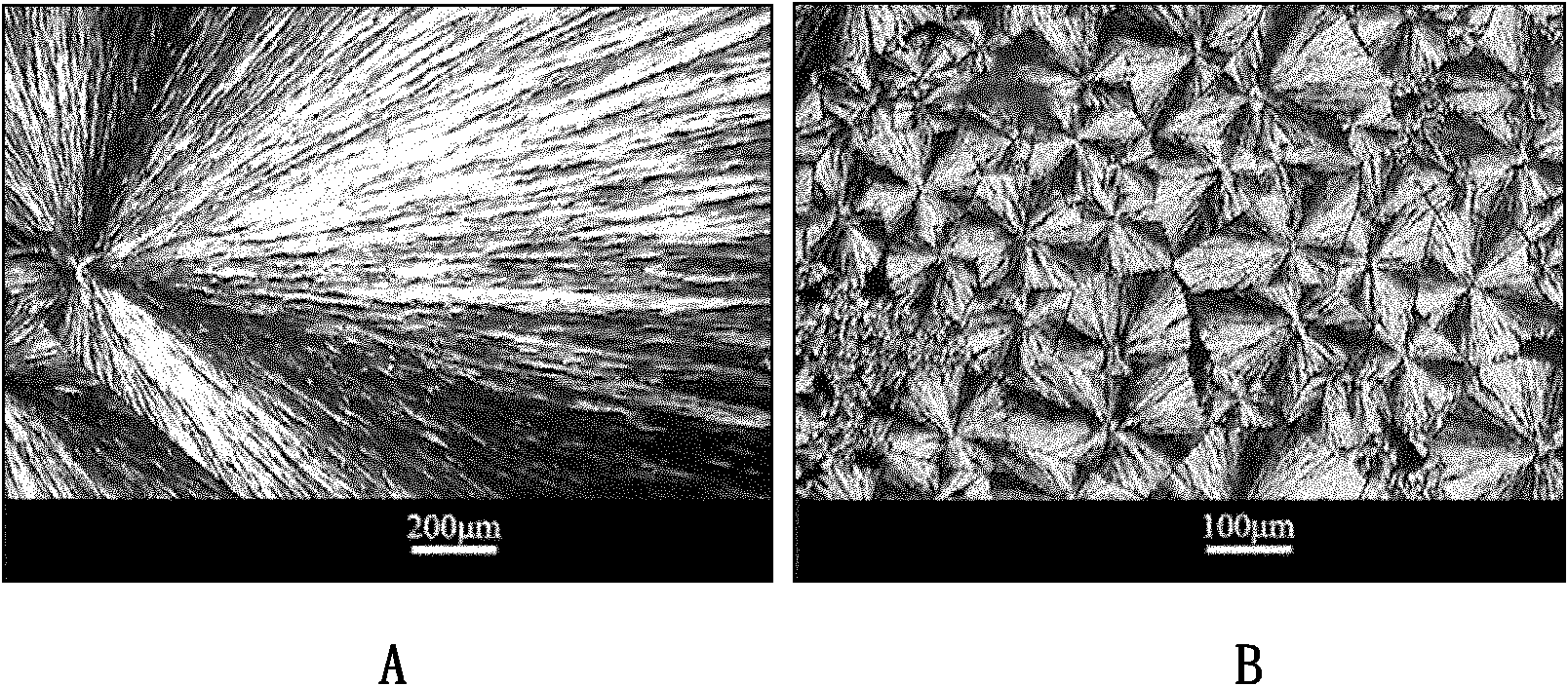
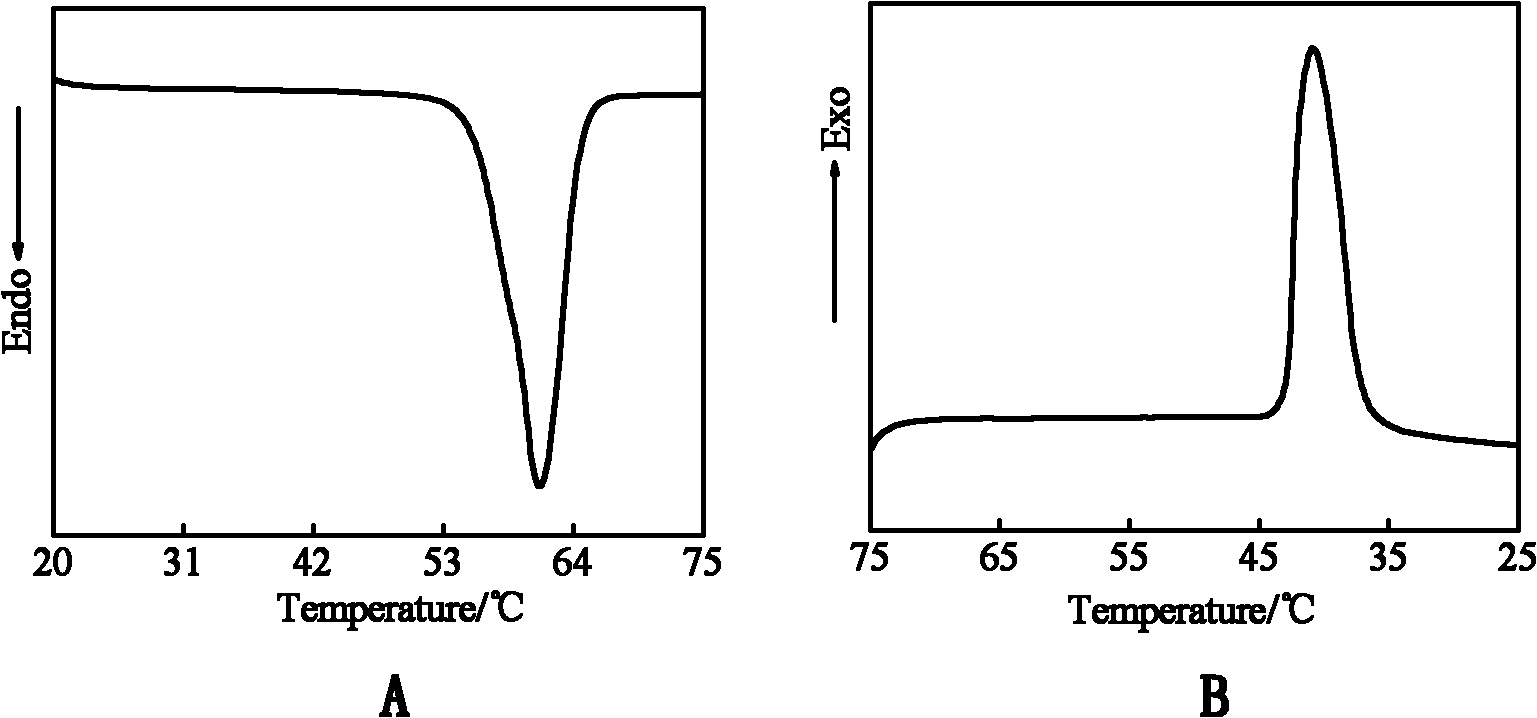
[0035] By changing the crystalline form of the phase change monomer (see figure 2 ) observed that polyethylene g...
Embodiment 2
[0040] In an ice-water bath, dissolve 10 g of polyethylene glycol with a molecular weight of 4000 in 30 ml of dichloromethane, add 0.8 g of triethylamine, and slowly add 0.7 g of acryloyl chloride; React for 4 hours; put the reaction solution in an ice-water bath, add 150ml of ether, let it stand for 10-15 minutes, and filter under reduced pressure to obtain the phase-change monomer polyethylene glycol acrylate with double bond end groups.
Embodiment 3
[0042] In an ice-water bath, dissolve 10 g of polyethylene glycol with a molecular weight of 2000 in 20 ml of dichloromethane, add 0.4 g of N, N-dimethylacetamide, and slowly add 0.5 g of acryloyl chloride dropwise; raise the temperature of the system to 35 ℃, under the protection of nitrogen for 8 hours; put the reaction solution in an ice-water bath, add 150ml of n-hexane, let it stand for 10-15 minutes, and filter under reduced pressure to obtain the phase-change monomer polyethylene glycol with double bond end groups Acrylate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com