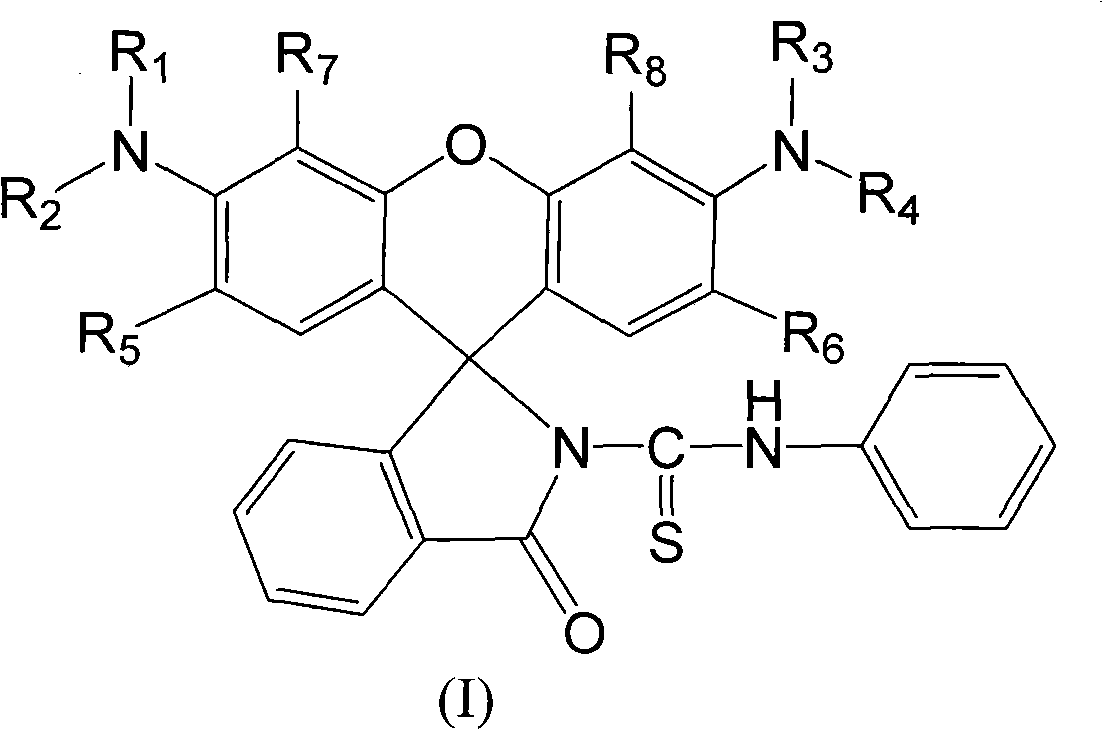
N-(rhodamine 6G) lactam-N'-phenylthiourea derivative fluorescent probe and preparation method
A technology of phenylthiourea and lactam, applied in chemical instruments and methods, luminescent materials, triarylmethane dyes, etc., to achieve the effect of accelerating the dissolution rate and enhancing the stability of pH value
Inactive Publication Date: 2011-06-15
QINGDAO UNIV OF SCI & TECH
View PDF1 Cites 15 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
So far, the spiro rings in this type of compounds are mainly lactam and aromatic structures [Zhang Wenjuan, Wu Zengxi, et al. Spectroscopy and Spectral Analysis. 2010, 30(5): 1305; Li Honglin, Fan Jiangli, Liu Xiaojian, et al. Acta Chemical Sinica. 2010, 31(9): 1725. Zhang Lingfei, Zheng Xiangyong, Zeng Xi, et al. Acta Inorganic Chemistry. 2010, 26(7): 1183.], but the rhodamine in which the hydrogen in the imine group of the spiro ring is replaced Derivatives and their preparation methods are seldom reported
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
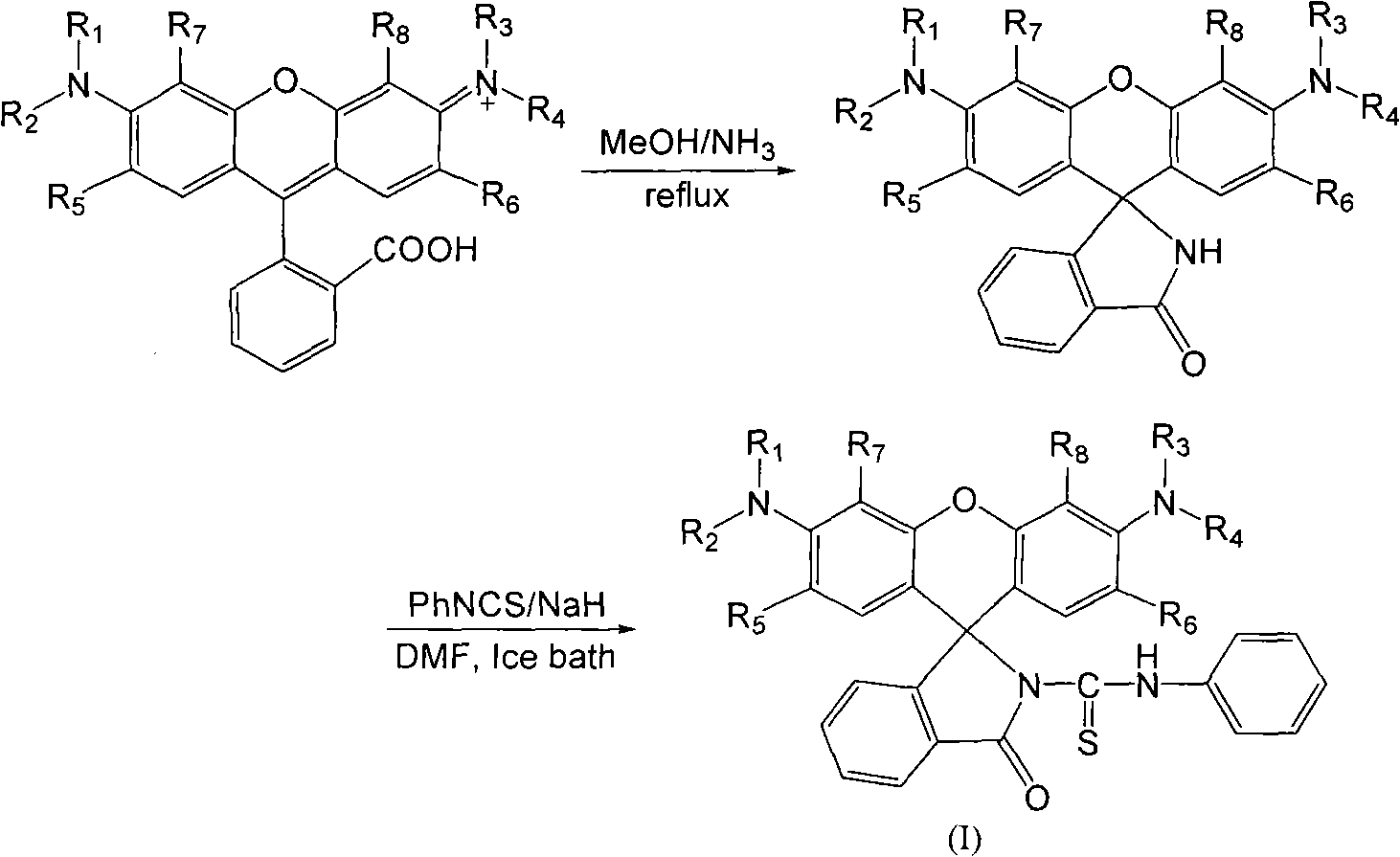
The invention discloses an N-(rhodamine 6G) lactam-N'-phenylthiourea derivative. The N-(rhodamine 6G) lactam-N'-phenylthiourea derivative has the chemical structural formula shown as the specifications. The invention also discloses a preparation method of the N-(rhodamine 6G) lactam-N'-phenylthiourea derivative. The preparation method comprises the following steps of: reacting rhodamine 6G serving as a raw material with ammonia to prepare rhodamine 6G lactam; and reacting the rhodamine 6G lactam with phenylisothiocyanate to prepare the N-(rhodamine 6G) lactam-N'-phenylthiourea derivative. A fluorescent probe has the characteristics of increasing dissolution velocity in acetonitrile, enhancing stability of the pH value; stabilizing in acid alkaline environments and the like, and can serves as a probe for a fluorescent indicator of metal ions to detect heavy metal ion content and transition metal ion content of water.
Description
A kind of N-(rhodamine 6G) lactam-N'-phenylthiourea derivative fluorescent probe and preparation method thereof Technical field: The invention relates to a fluorescent probe-N-(rhodamine 6G) lactam-N'-phenylthiourea derivative and a preparation method thereof. Background technique: Rhodamine series compounds are important members of the xanthene dye family. The absorption and emission are at longer wavelengths, the fluorescence quantum yield is high, the extinction coefficient is large, and the photostability is good. It has attracted the attention of scientists for a long time. Due to its excellent optical properties, it is often used as a fluorescent probe for detecting trace metal ions. The selective recognition of metal ions by rhodamine-based dyes is through the complexation of metal ions and probe molecules, which induces the opening of the helical ring structure of the probe, thereby generating fluorescent signals. In view of this, long-wavelength fluorescent switc...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D491/107C09K11/06
CPCC09B11/24
Inventor 胡志强詹天荣王磊
Owner QINGDAO UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com