Preparation method of cholesterol derivative-based organic-inorganic composite nano vesicle
A cholesterol derivative, inorganic compound technology, applied in the directions of non-active ingredients medical preparations, liposome delivery, pharmaceutical formulations, etc., can solve the limitation of clinical application and industrial production of liposome preparations, in vivo stability and storage stability problems such as poor biocompatibility and improved stability, achieving good application prospects, overcoming poor biocompatibility and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of Cholesterol Succinate Monoester: Cholesterol (1eq.), succinic anhydride (3eq.) and DMAP (0.1eq.) were dissolved in dichloromethane and refluxed at 55°C for 3 days. Solvent was removed under vacuum. The residue was dissolved in ethanol and poured into cold 15% NaCl solution. The resulting suspension was adjusted to pH 2 with 1M HCl. Filter and wash with neutral water. The dried solid was recrystallized from ethanol / ethyl acetate (10:1, v / v) in nearly 100% yield.
[0038] Synthesis of organic-inorganic composite cholesterol derivatives (1): as figure 1 As shown, EDC (1.2eq.) and cholesterol succinic acid monoester (1.2eq.) were mixed and dissolved in dry dichloromethane. After 30min, APTES (1.2eq.) was added and stirred at room temperature for 4h. The solvent was removed in vacuo, and the crude product was separated with a silica gel column using ethyl acetate / dichloromethane (1:3 v / v) as the developing solvent to obtain a white solid with a yield of abou...
Embodiment 2
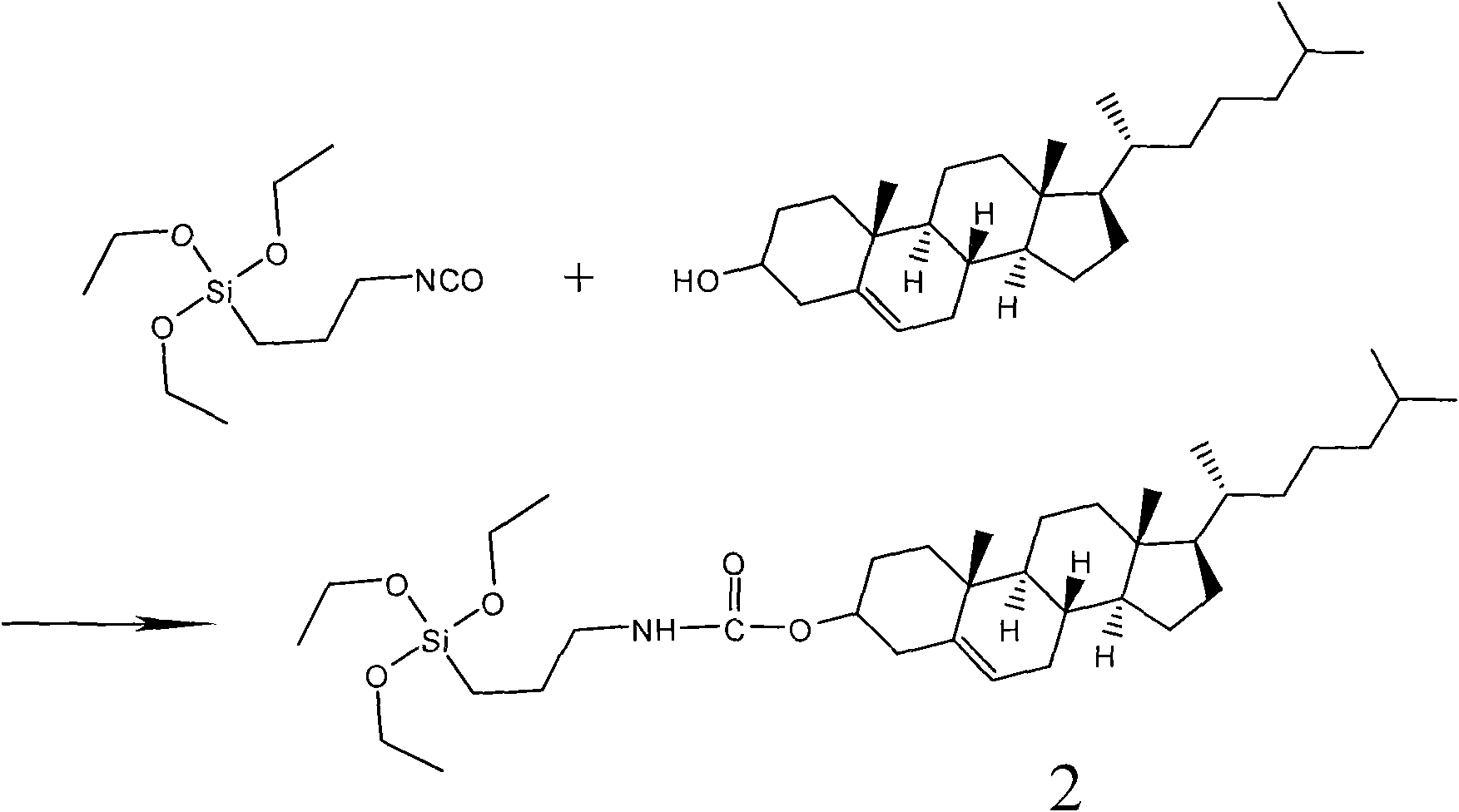
[0040] Synthesis of Organic-Inorganic Complex Cholesterol Derivatives (2): Such as figure 2Cholesterol (0.5mmol) was dissolved in 20mL of dry dichloromethane as shown, and then added isocyanatopropyltriethoxysilane (IPTES, 0.5mmol) and dibutyltin dilaurate (0.1mmol ), the reaction mixture was stirred at 50° C. for 24 h under nitrogen protection, and the progress of the reaction was monitored by TLC. After the reaction was completed, the solvent was removed under vacuum, and the crude product was separated with a silica gel column using ethyl acetate / n-hexane (1:5 v / v) as the developing solvent to obtain a white solid with a yield of about 75%. The melting point measured by the melting point instrument is 81-82°C; the NMR results are as follows: 1 H NMR (CDCl 3 , 400MHz) δ: 0.63 (t, J=8.0Hz, 2H, NHCH 2 CH 2 CH 2 Si), 0.67(s, 3H, CH 3 ), 0.85~0.87 (m, 6H, CH 3 ), 0.90~0.92 (m, 3H, CH 3 ), 1.00 (s, 3H, CH 3 ), 1.02~1.21(m, 6H), 1.22~1.35(m, 9H), 1.35~1.47(m, 3H), 1.48~1...
Embodiment 3
[0042] The synthesized organic-inorganic composite cholesterol derivative (1) was dissolved in ethanol, and then injected into the aqueous solution under ultrasonic conditions to allow self-assembly to prepare vesicles with a liposome-like structure. DLS results showed that its The particle size is about 100-200nm, which is consistent with the results of TEM and SEM. EDX results showed that the surface contained C, O, and Si elements, indicating that the surface of the vesicle had a Si-O-Si structure ( image 3 ), demonstrating the formation of a silica shell on its surface.
[0043] The stability of CSS vesicles was measured by measuring the size change of CSS vesicles by adding Triton X-100. the result shows( Figure 4 ), when TritonX-100 was added to the DPPC and CHS liposome suspension, it would initially cause the particle size of the vesicles to increase and then suddenly decrease, which indicated that the vesicles had been destroyed. In contrast, the particle size of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com