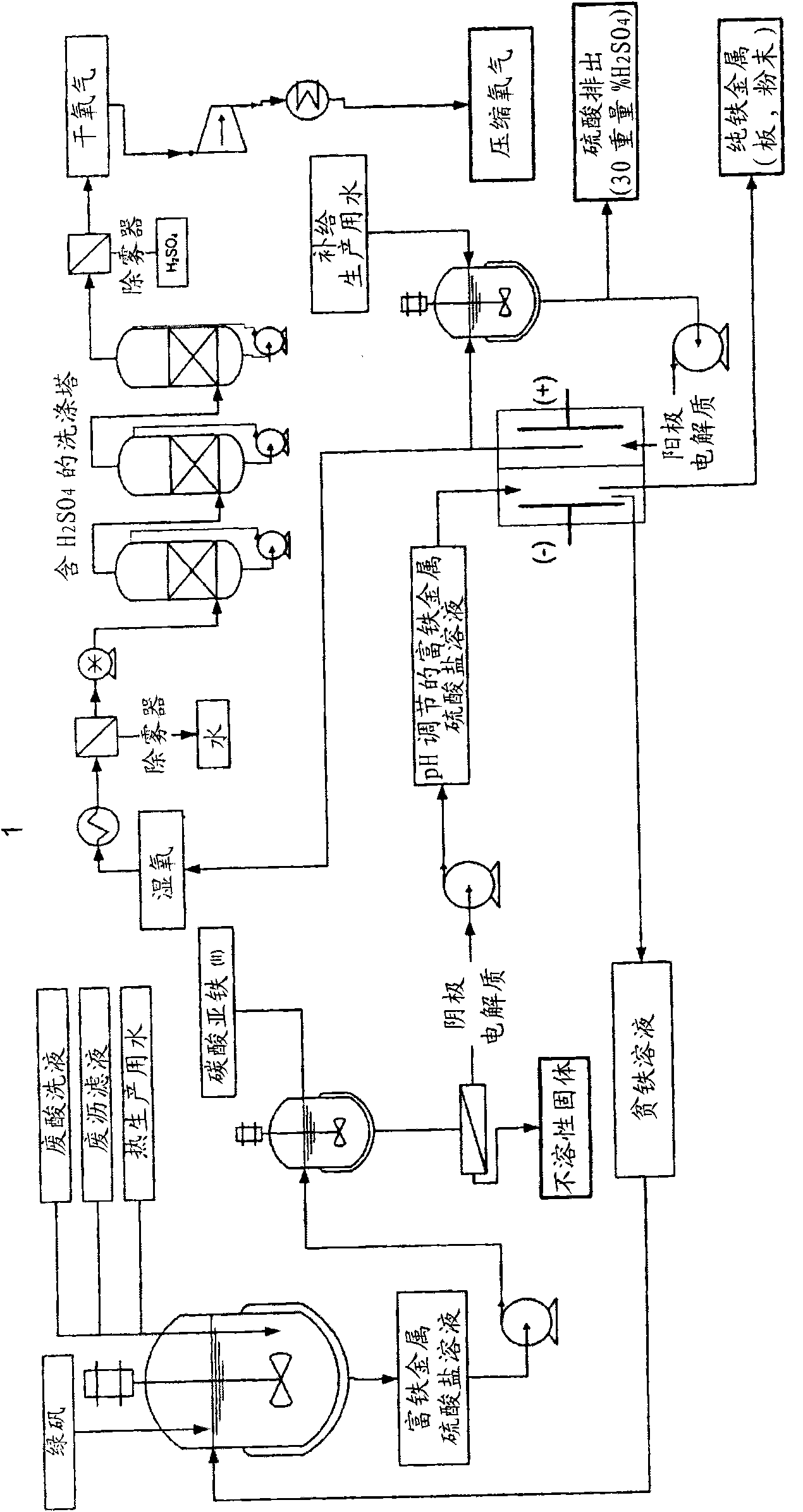
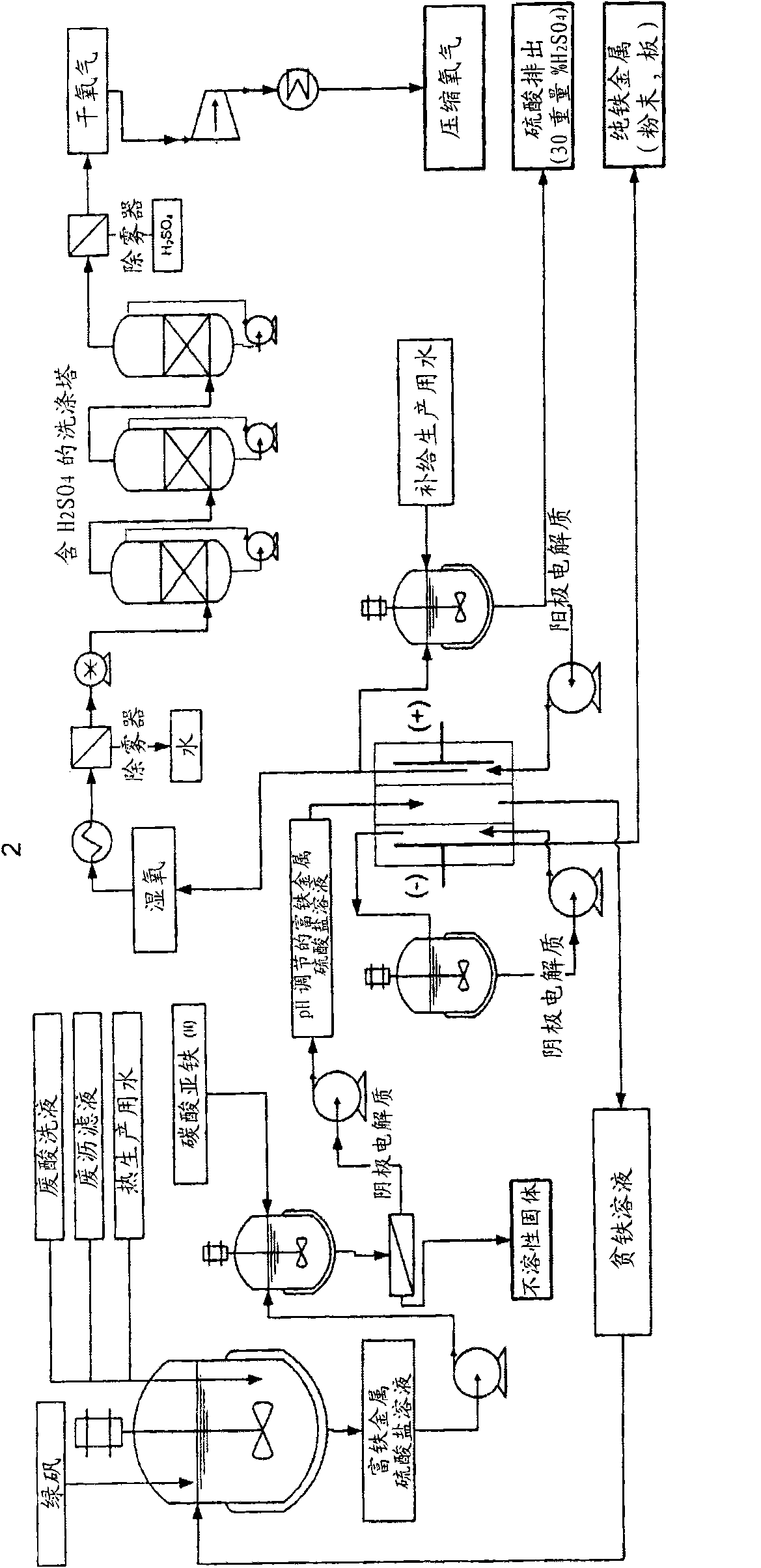
Electrochemical process for the recovery of metallic iron and sulfuric acid values from iron-rich sulfate wastes, mining residues and pickling liquors
An electrochemical and metal sulfate technology, applied in the direction of electrodes, optics, electrolysis, etc., can solve the problems of expensive organic solvents, and the scale of industrial application has not been reached.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Preparation of iron-rich metal sulfate solutions and removal of trace ferric cations. A batch of iron (II) sulfate heptahydrate, also known as columbite, from a titanium pigment producer was used to prepare the synthesis solution. The material was dissolved in deionized and degassed water. After the soluble salts were completely dissolved, samples were taken for measurement of mass density, total iron content and concentration of ferric cations.
[0129] After determining the ferric cation, the pH of the solution was adjusted by adding iron (II) carbonate or sulfuric acid until the pH of the solution reached 3.5. At this pH, any traces of ferric iron were deposited as ferric hydroxide, which was subsequently removed by filtration. The clarified ferric sulfate solution is then acidified to a pH of about 0.5 (at this pH, air oxidation of ferrous iron (Fe 2+ ) into ferric iron (Fe 3+ )the process of). A nitrogen blanket was maintained over the solution to further prev...
Embodiment 2
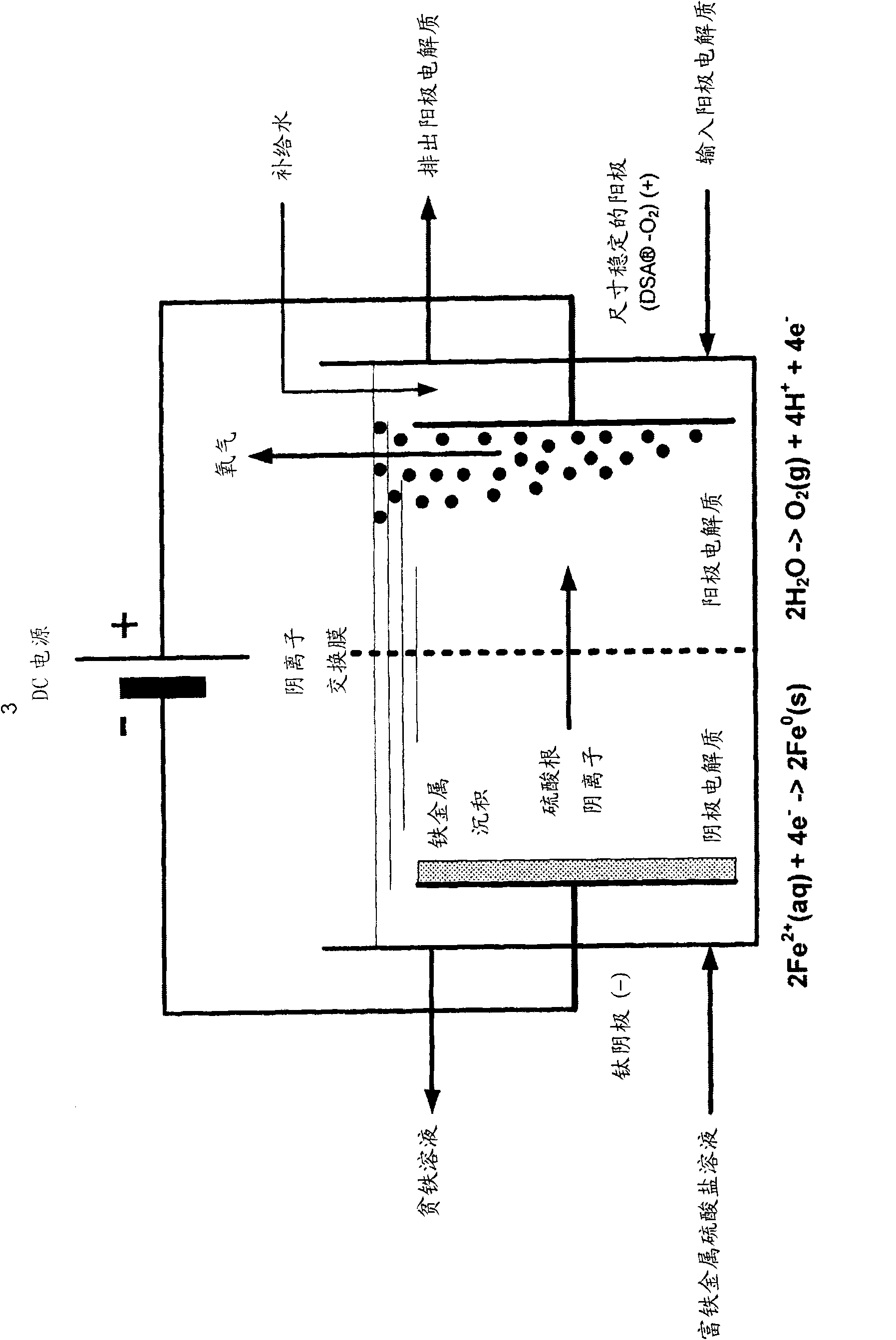
[0131] Example 2a - Electrolysis of iron rich metal sulphate solution at pH 1.4 and 50°C. The pH value of the iron-rich metal sulfate solution of Example 1 was adjusted to 1.4 by adding a trace amount of iron (II) carbonate, and then circulated inside the cathode chamber of the electrolytic cell. The electrolytic cell consists of a plate type electrolytic cell ( Figure 5 ) and two compartments separated by an anion exchange membrane. Geometry Electrode and membrane surface area is 929cm 2 (1 square foot) with a 1 inch (2.54 cm) separation between each electrode and separator. The cathode chamber contained the cathode plate, which was made of CP titanium (ASTM grade 2, supplied by RMI (Niles, OH)). Before electrolysis, by immersing the cathode in boiling oxalic acid (10 wt% H 2 C 2 o 4 ) and rinsed thoroughly with deionized water until no traces of acid remained. The anode compartment is filled with dimensionally stable TiR- type anode (DSA TM -O 2 ) (supplied by El...
Embodiment 3
[0141] The iron-rich metal sulfate solution was electrolyzed using a three-compartment electrolytic cell. The pH value of the iron-rich metal sulfate of Example 1 was adjusted to 1.4 by adding iron (II) carbonate, and then circulated inside the central chamber of the three-chamber electrolytic cell. The electrolytic cell consists of a plate type electrolytic cell ( Figure 4 ) consists of three compartments separated by an anion exchange membrane and a cation exchange membrane. Geometry Electrode and membrane surface area is 929cm 2 (1 square foot) with a 1 inch (2.54 cm) separation between each electrode and separator. The spacing between individual films was 1 inch (2.54 cm).
[0142] The cathode chamber contained the cathode plate, which was made of CP titanium (ASTM grade 2, supplied by RMI (Niles, OH)). Before electrolysis, by immersing the cathode in boiling oxalic acid (10 wt% H 2 C 2 o 4 ) and rinsed thoroughly with deionized water until no traces of acid remain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com