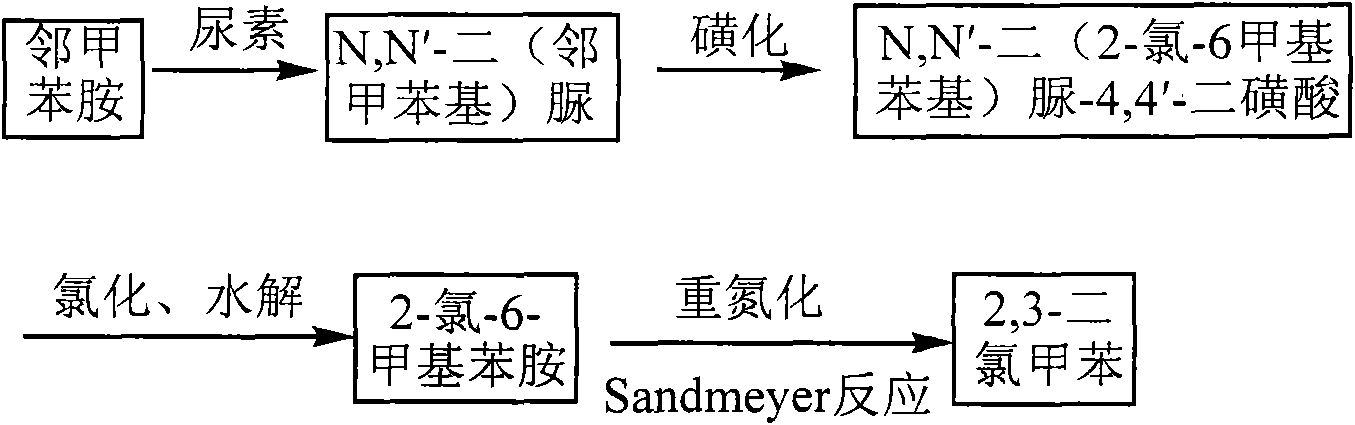
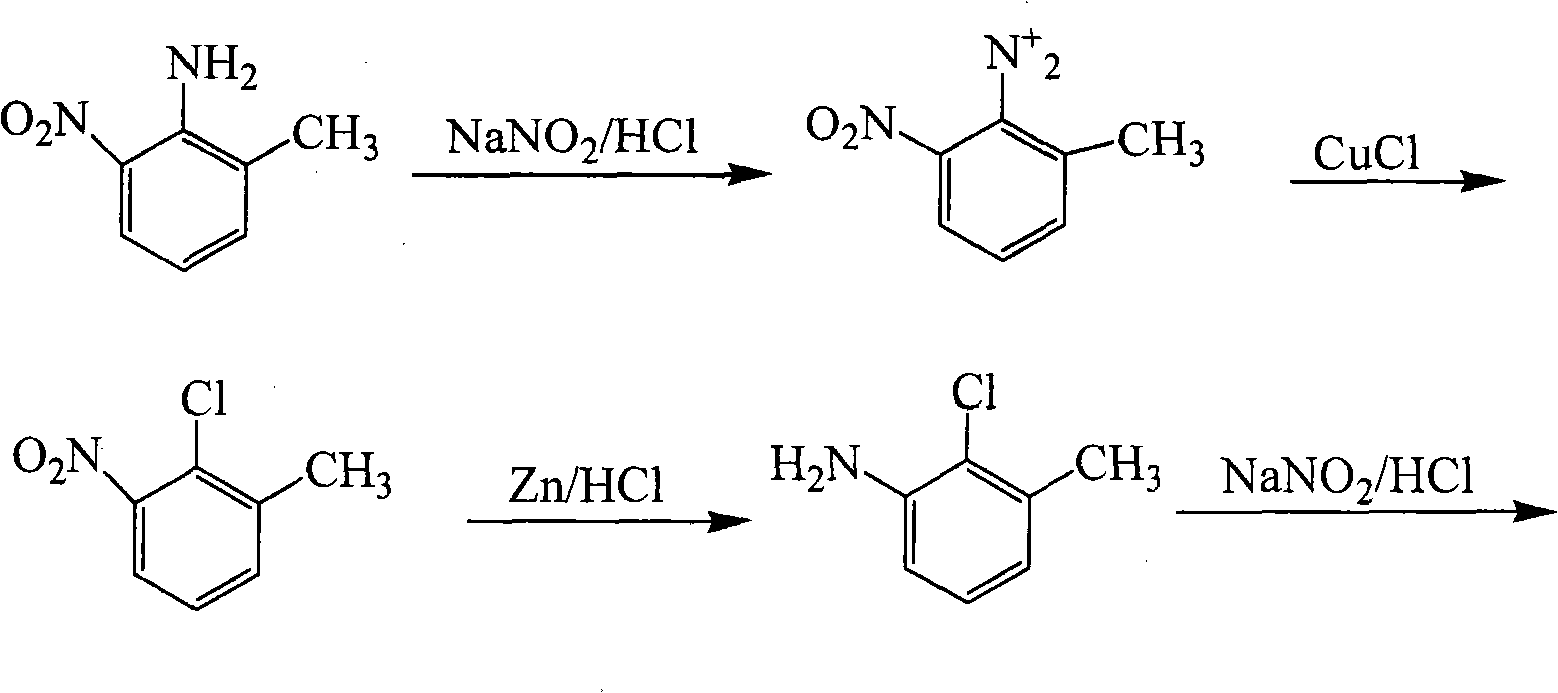
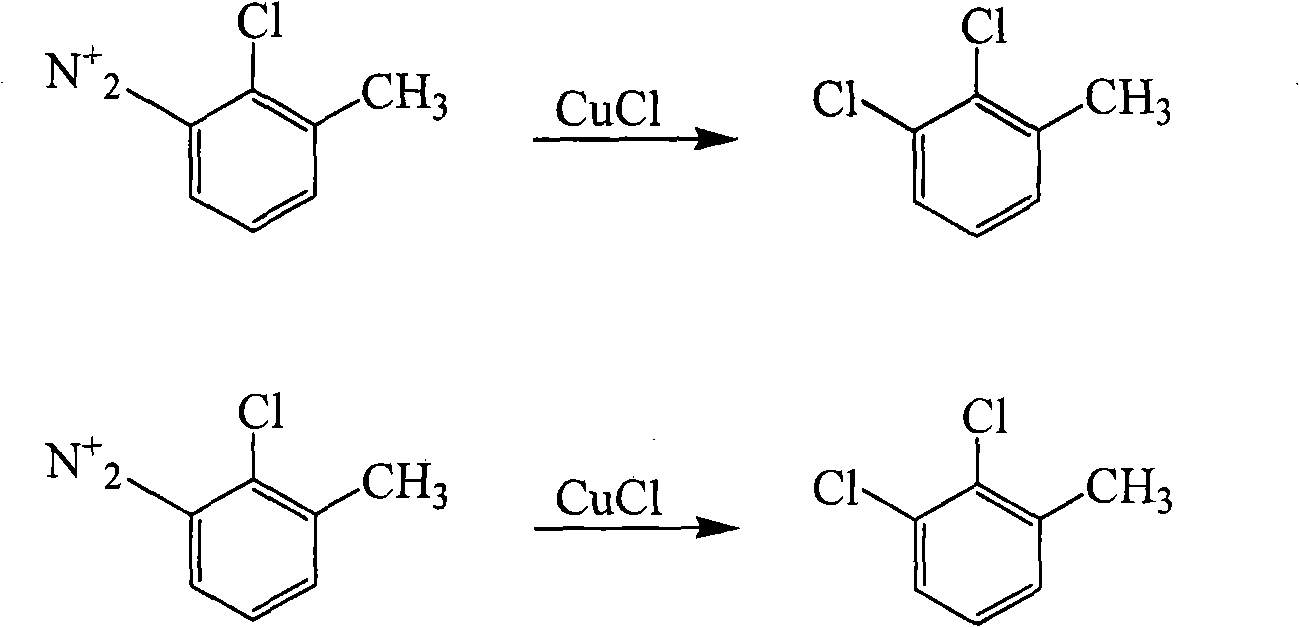
Method for preparing 2,3-dichlorotoluene
A dichlorotoluene and o-tolyl-based technology, applied in 2 fields, can solve the problems of inaccessibility of raw materials, high price, long time-consuming and the like of the synthetic route, and achieve the effects of low cost, low price and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Add o-toluidine 43ml, urea 18g, and xylene 170ml in the 500ml round-bottomed three-neck flask that stirring, measuring temperature and reflux device are housed and heat up to 138 ℃, reflux reaction 7 hours, pour out while hot. Then cool to normal temperature under stirring, filter, the filter cake is washed with xylene, drained, and dried, and then washed with a large amount of water, drained, and dried to obtain 42.90 g of white needle crystals, in terms of o-toluidine, N, The yield of N'-di(o-tolyl)urea was 89.40%.
[0029] Pour 67mL of sulfuric acid into a 500mL three-necked flask equipped with a thermometer and an exhaust gas recovery device, weigh 24.00g of N,N'-di(o-tolyl)urea, add it to the solution in batches under stirring, and the addition temperature does not exceed 50°C , after the N,N'-di(o-tolyl)urea was completely dissolved, the temperature was kept at 65° C. for 6 hours, and then the heating was stopped. This gives N,N'-di(o-tolyl)urea-4,4...
Embodiment 2
[0031] Embodiment 2: Weigh 24.00 N, N'-bis(o-tolyl)urea and put it into a 1000ml three-necked flask, pour 10% oleum of 62ml into the dropping funnel, add dropwise and the temperature does not exceed 50°C , dissolve N, N'-bis(o-tolyl)urea, wait until N,N'-bis(o-tolyl)urea is completely dissolved, keep the temperature at 65°C for 6 hours, stop heating. Get N,N'- Bis(o-tolyl)urea-4,4'-disulfonic acid. Add 300ml of water, 160ml of dilute hydrochloric acid and 0.56g of iron powder, pass chlorine gas, keep the heating temperature at 35°C, and react for 12h. To obtain N,N'-bis(2-chloro-6-methylphenyl)urea-4,4'-disulfonic acid, assemble a reflux device, pass nitrogen protection, slowly raise the temperature of the reaction solution to 107°C, and heat-preserve and hydrolyze for 3h Afterwards, the solution was heated to 175° C. in an autoclave. React for 10 hours. After cooling, use sodium carbonate to neutralize to neutrality, then carry out steam distillation, collect the distillat...
Embodiment 3
[0032] Embodiment 3: Weigh 24.00 N, N'-bis(o-tolyl)urea and put it into a 1000ml three-necked flask, pour 67ml of 98% concentrated sulfuric acid into the dropping funnel, add dropwise and the temperature does not exceed 50°C, Dissolve N,N'-di(o-tolyl)urea, wait until N,N'-bis(o-tolyl)urea is completely dissolved, keep the temperature at 65°C for 6 hours, stop heating. Obtain N,N'-di(o-tolyl)urea (o-tolyl)urea-4,4'-disulfonic acid. Add 300ml of water, 160ml of dilute hydrochloric acid and 0.56g of iron powder, pass chlorine gas, keep the heating temperature at 35°C, and react for 12h. To obtain N,N'-bis(2-chloro-6-methylphenyl)urea-4,4'-disulfonic acid, assemble a reflux device, pass nitrogen protection, slowly raise the temperature of the reaction solution to 107°C, and heat-preserve and hydrolyze for 3h Afterwards, the solution was heated to 175° C. in an autoclave. React for 10 hours. After cooling, use sodium carbonate to neutralize to neutrality, then carry out steam di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com