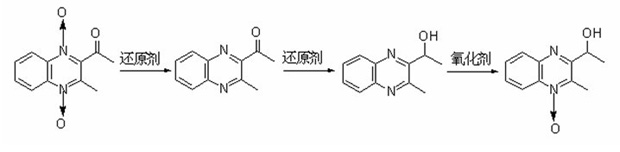
1-oxo-2-methyl-3-(1-ethoxyl)-quinoxaline and preparation method and application thereof
A quinoxaline and hydroxyethyl technology, which is applied in the field of 1-oxo-2-methyl-3-(1-hydroxyethyl) quinoxaline and its preparation, can solve rough detection and cannot accurately reflect acetyl methyl Quine metabolism, inability to accurately reflect the residual content of methaquine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 7.5 grams of acemetquine and 200 milliliters of 60 volume % ethanol aqueous solution to the reactor respectively, add 50 grams of sodium dithionite in batches to react at 80 ° C within half an hour, stir and react at 80 ° C for 3 hours, filter, Purified to obtain 3-methyl-2-(acetyl)-quinoxaline with a yield of 88%;
[0040] Add 1.2 grams of 3-methyl-2-(acetyl)-quinoxaline and 12 milliliters of 95% by volume ethanol aqueous solution to the reactor respectively, add 0.4 grams of sodium cyanoborohydride at 25°C, and react at 25°C After 1 hour, the solvent was removed, washed, extracted, and dried to obtain 2-(1-hydroxyethyl)-3-methyl-quinoxaline with a yield of 95%;
[0041]Add 1.2 grams of 3-methyl-2-(1-hydroxyethyl)-quinoxaline and 1.2 grams of m-chloroperoxybenzoic acid to the reactor and mix them at 0°C, then slowly rise to 25°C after 15 minutes to continue React for 8 hours, add 30 milliliters of 10% by weight sodium carbonate solution after the reaction is finis...
Embodiment 2
[0043] Add 7.5 grams of acemethaquine and 200 milliliters of 80 volume % ethanol aqueous solution to the reactor respectively, add 60 grams of sodium dithionite in batches to react within half an hour at 100 ° C, stir and react at 80 ° C for 5 hours, filter, Purified to obtain 3-methyl-2-(acetyl)-quinoxaline with a yield of 72%;
[0044] Add 1.2 grams of 3-methyl-2-(acetyl)-quinoxaline and 12 milliliters of 95% by volume tetrahydrofuran aqueous solution to the reactor respectively, add 0.54 grams of sodium cyanoborohydride at 25°C, and react at 25°C After 0.5 hour, the solvent was removed, washed, extracted, and dried to obtain 2-(1-hydroxyethyl)-3-methyl-quinoxaline with a yield of 99%.
[0045] 1.2 grams of 3-methyl-2-(1-hydroxyethyl)-quinoxaline and 1.0 grams of peroxybenzoic acid were added to the reactor and mixed at 0°C. After 15 minutes, it was slowly raised to 25°C to continue the reaction for 12 After the reaction was completed, 30 milliliters of 10% by weight sodium...
Embodiment 3
[0050] After 4 hours of intramuscular injection of acemethaquine in healthy pigs, blood samples were collected, extracted 3 times with organic solvents, blown dry with nitrogen, and added mobile phase to make a sample solution; another blood sample from untreated healthy pigs was prepared as a blank control according to the above method ; Take compound (I) and add mobile phase to make 1mg / mL reference substance solution.
[0051] High-performance liquid chromatography: UltiMate3000 American Diane Company; chromatographic conditions: Hypersil BDS C 18 Chromatographic column, 250 mm×4.6 mm×5 μm, column temperature: 30 ℃; mobile phase: methanol (B) / water (containing 0.01% formic acid) (A), gradient elution: 0-40 min, 20%-35 % B; 40-45 min, 35%-60% B; 45-57 min, 60% B; 57-60 min, 60%-20% B; 60-65 min, 20% B; flow rate: 1 mL / min, detection wavelength: 241 nm.
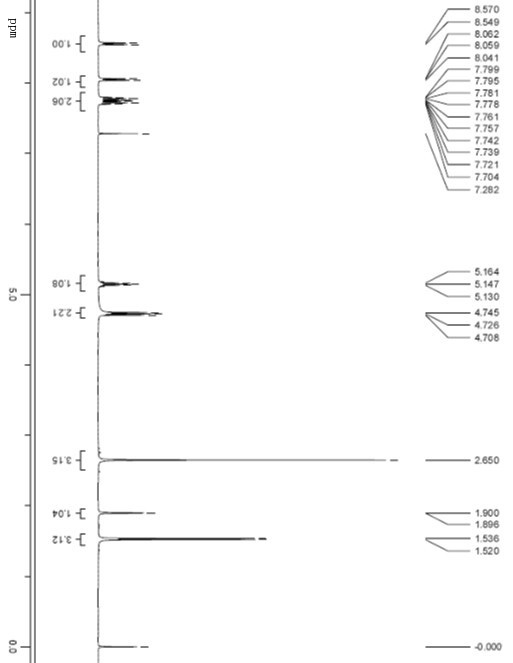
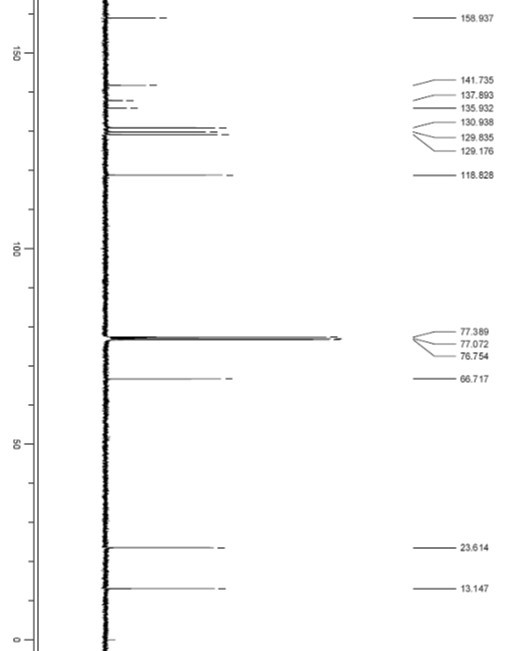
[0052] The retention time of compound I was 19.87min, the peak appeared in the sample solution at 19.93min, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com