Method for preparing halofuginone intermediate
An intermediate, the technology of hemosanone, which is applied in the field of preparation of hemosanone intermediates, can solve the problems of difficult to complete the reaction, unfavorable large-scale preparation, and difficult post-processing, so as to improve efficiency, reduce the amount of solvent used, and reduce adverse effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 compound G
[0048]
[0049] N 2 Under protection, 1.9g (20mmol) of 3-hydroxypyridine was added to 15ml of toluene, and then 2.4ml (21mmol) of benzyl chloride was added at one time, heated to reflux, the insoluble matter gradually disappeared, and the solution was clear. Along with the progress of the reaction, an oily liquid was generated at the bottom of the solution, heated and refluxed for 1 hour, stopped heating, cooled, the oily liquid at the bottom became a solid, poured out the upper layer solution, and the solid was ground with a mortar, then washed three times with ethyl acetate, It was then washed once with ether. After vacuum drying, 4.0 g of compound G was obtained with a yield of 91%, melting point: 159-161°C (literature value: 159-160°C).
Embodiment 2
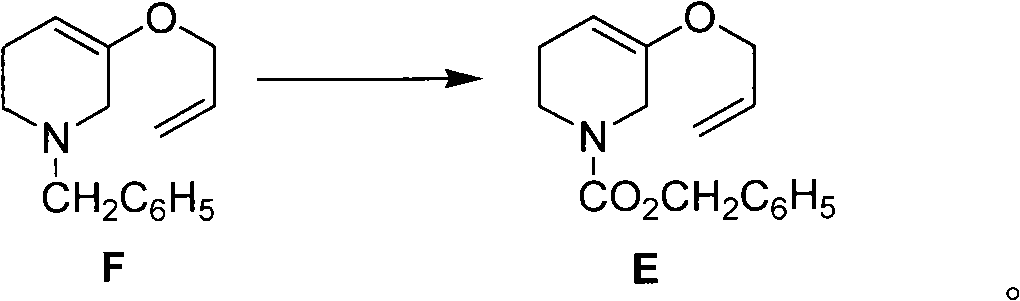
[0050] The preparation of embodiment 2 compound F
[0051]
[0052] N 2 Under protection, 3.8g (17mmol) of compound G was dissolved in 10ml of methanol, 1.6ml (19mmol) of 3-bromopropene was added, 0.60g (20mmol) of 80% sodium hydride was weighed, added to the above solution in batches, and refluxed for 4 hours , cooled to 0°C, added 0.72g (19mmol) sodium borohydride in batches, and kept at 0°C for 30 minutes. After the reaction, adjust the pH to 5-6 with 3mol / l hydrochloric acid, then adjust the pH to 7-8 with saturated sodium bicarbonate solution, extract with ethyl acetate (10ml×4), combine the organic phases, wash with saturated brine three times, After drying and evaporating the solvent, 2.4 g of the crude product was obtained, which was quickly eluted with a simple short silica gel column (eluent: petroleum ether) to obtain 2.3 g of a colorless oily liquid with a yield of 60%. The structural identification data are as follows: 1 H NMR (CDCl 3 ): δ2.16~2.19(2H, m), 2...
Embodiment 3
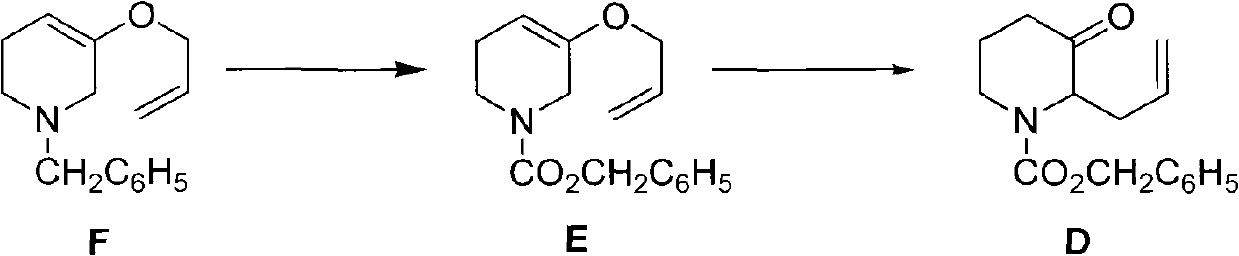
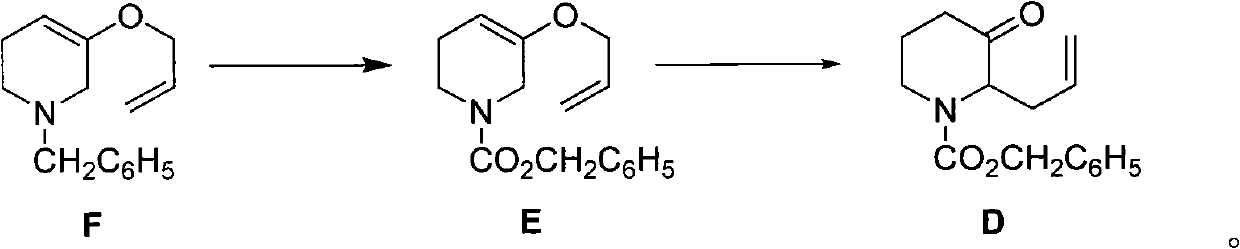
[0053] The preparation of embodiment 3 compound D
[0054]
[0055] N 2 Under protection, 6ml (42mmol) of benzyl chloroformate was dissolved in 6ml of tetrahydrofuran, and at 0°C, 2.40g (10.5mmol) of compound F in 6ml of tetrahydrofuran was added dropwise thereto. room temperature and stirred for another 2 hours. Slowly raise the temperature to reflux, recover tetrahydrofuran by atmospheric distillation, then recover about 4ml of benzyl chloroformate by distillation under reduced pressure, and purify the residue by silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 20:1) to obtain light yellow Viscous liquid 2.5g, two-step reaction total yield 70%, the structural identification data of the product are as follows: 1 H NMR (CDCl 3 ): δ1.88~1.93(2H, m), 2.41~2.52(2H, m), 3.22~3.26(1H, m), 4.07~4.19(1H, m), 4.58~4.69(1H, m), 5.04 ~5.15 (4H, m), 5.15 (2H, s), 5.51 ~ 5.75 (1H, m), 7.29 ~ 7.36 (5H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com