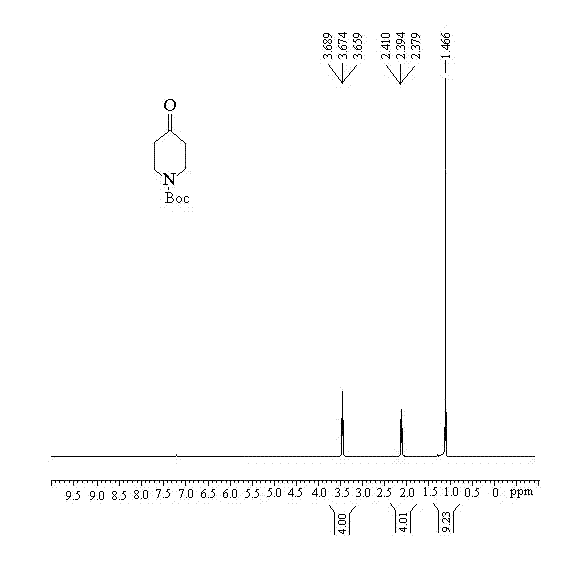
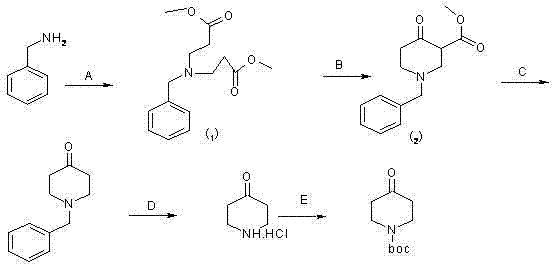
Synthesis method of 1-teriary butoxy carbonyl-4-piperidone
A technology of tert-butoxycarbonyl and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of backward production process, low yield, low purity of 1-tert-butoxycarbonyl-4-piperidone, etc., and reduce energy consumption. , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] According to the molar ratio of 1:2, benzylamine and methyl acrylate were taken respectively, methyl acrylate and benzylamine were dissolved in methanol, and Michael addition reaction was carried out at room temperature for 10 hours, and then, the product of the reaction was purified by the existing method, After the purification is completed, methanol is distilled off at a temperature of 40° C. to obtain piperidone intermediate 1; according to a molar ratio of 1.2:1, metal sodium and prepared piperidone intermediate 1 are respectively taken, and piperidone intermediate 1 is prepared. Dissolve metal sodium in toluene, conduct Dickmann condensation reaction at 100°C for 2 hours, purify the product, and obtain piperidone intermediate 2; the molar ratio is 6:1, and the concentration is 5mol / L Concentrated hydrochloric acid and the obtained piperidone intermediate 2 were decarboxylated at 80°C for 4 hours to obtain the crude product 1-benzyl-4-piperidone hydrochloride; and ...
Embodiment 2
[0038] According to the molar ratio of 1:2.5, take benzylamine and methyl acrylate respectively, dissolve methyl acrylate and benzylamine in methanol, carry out Michael addition reaction at room temperature for 16 hours, then purify the reaction product by using existing methods, purify After completion, methanol was distilled off at a temperature of 60° C. to obtain piperidone intermediate 1; according to a molar ratio of 2:1, metal sodium and prepared piperidone intermediate 1 were respectively taken, and piperidone intermediate 1 was prepared. Dissolve metal sodium in toluene, and conduct a Dickmann condensation reaction at 125°C for 3 hours, then purify the product to obtain piperidone intermediate 2; the molar ratio is 12:1, and the concentration is taken separately Concentrated hydrochloric acid of 7 mol / L and the prepared piperidone intermediate 2, decarboxylation reaction at 100°C for 8 hours to obtain crude product 1-benzyl-4-piperidone hydrochloride; volume ratio 1:3 ...
Embodiment 3
[0040] According to the molar ratio of 1:2.25, benzylamine and methyl acrylate were taken respectively, methyl acrylate and benzylamine were dissolved in methanol, and Michael addition reaction was carried out at room temperature for 13 hours, and then, the product of the reaction was purified by the existing method, After the purification is completed, methanol is distilled off at a temperature of 50° C. to obtain piperidone intermediate 1; according to a molar ratio of 1.6:1, metal sodium and prepared piperidone intermediate 1 are respectively taken, and piperidone intermediate 1 and metal sodium were dissolved in toluene, and at a temperature of 112.5°C, Dickmann condensation reaction was carried out for 2.5 hours, and then, the product was purified to obtain piperidone intermediate 2; the molar ratio was 9:1, and the concentration was respectively Concentrated hydrochloric acid of 6mol / L and the prepared piperidone intermediate 2, decarboxylation reaction at 90°C for 6 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com