Continuous acidolysis process for calcium hydrogen citrate
A calcium hydrogen citrate, acid hydrolysis technology, applied in the separation/purification of carboxylic acid compounds, carboxylate preparation, organic chemistry, etc., can solve the problem of uncontrollable calcium sulfate particle size, incomplete acid hydrolysis reaction, acid hydrolysis solution problems such as low concentration, to achieve the effect of good energy saving and consumption reduction, easy comprehensive utilization and high concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
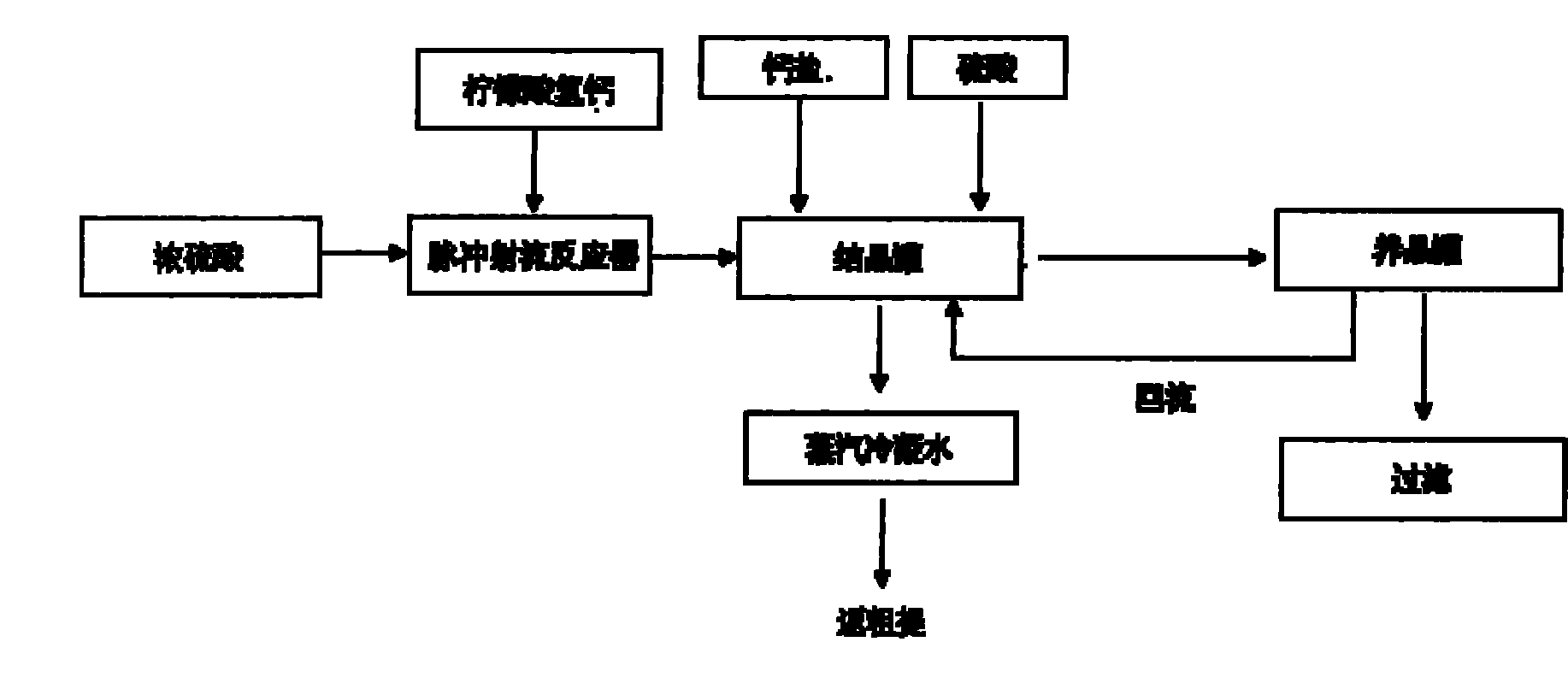
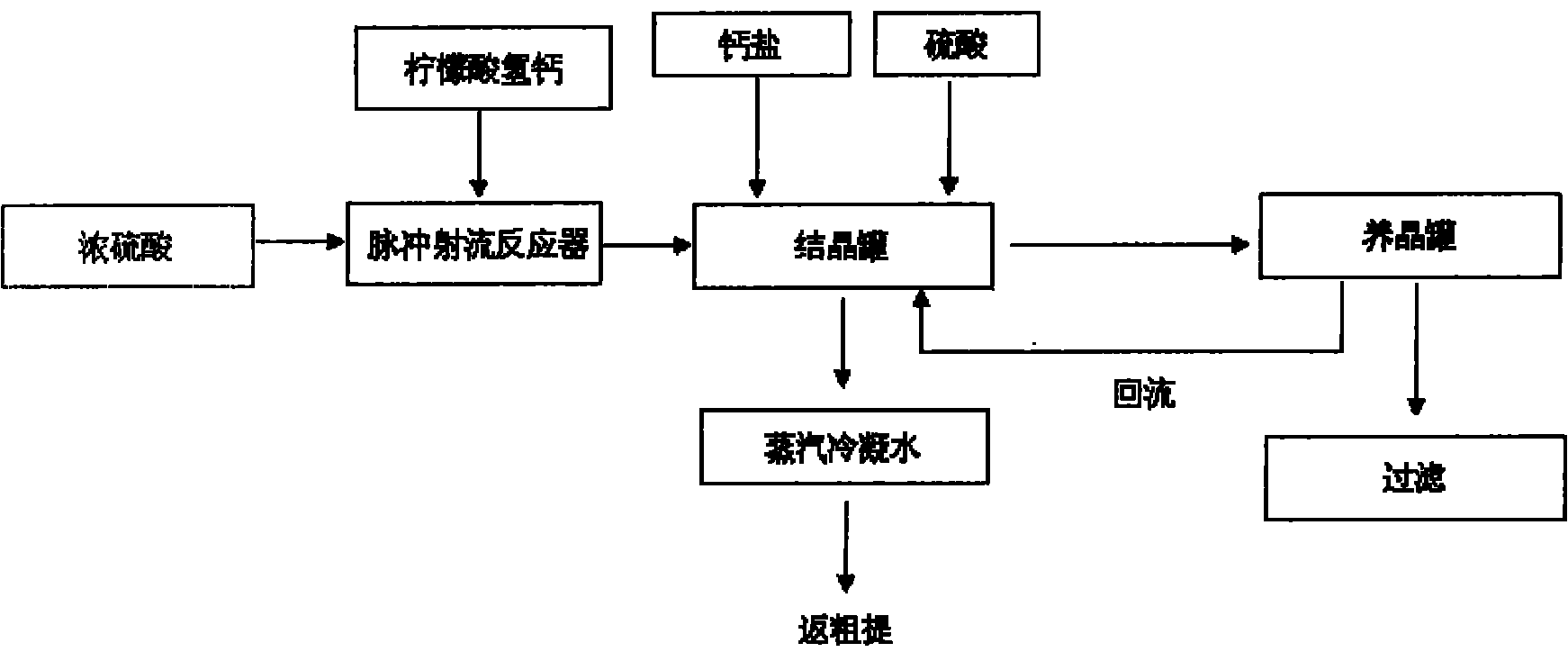
[0019] 1. Calcium hydrogen citrate and concentrated sulfuric acid complete the instantaneous acidolysis reaction through the pulse jet reactor on their respective pipelines. The concentration of concentrated sulfuric acid is 90%, the pH value is controlled at 1.5, and the reaction temperature is 80°C;
[0020] 2. Add calcium hydrogen citrate and sulfuric acid to the crystallization tank for the acid solution to fine-tune the pH value and complete the crystallization process. The pH value is controlled at 1.0, and the crystallization temperature is 83°C;
[0021] 3. The crystal slurry liquid is classified into calcium sulfate particles in the crystal growth tank, and the fine particles are returned to the crystallization tank with a reflux ratio of 0.3. Coarse particles of 100-110 mesh enter the filtration section to complete solid-liquid separation, and the acidity of the obtained acid hydrolysis solution is 51.12%. .
Embodiment 2
[0023] 1. Calcium hydrogen citrate and concentrated sulfuric acid complete the instantaneous acidolysis reaction through the pulse jet reactor on their respective pipelines. The concentration of concentrated sulfuric acid is 98%, the pH value is controlled at 1.4, and the reaction temperature is 88°C;
[0024] 2. Add calcium hydrogen citrate and sulfuric acid to the crystallization tank for the acidolysis solution to fine-tune the pH value and complete the crystallization process. The pH value is controlled at 2.5, and the crystallization temperature is 80°C;
[0025] 3. The crystal slurry liquid is classified into calcium sulfate particles in the crystal growth tank, and the fine particles return to the crystallization tank with a reflux ratio of 0.4. The coarse particles of 90-100 mesh enter the filtration section to complete the solid-liquid separation, and the acidity of the obtained acid hydrolysis solution is 52.42%. .
Embodiment 3
[0027] 1. Calcium hydrogen citrate and concentrated sulfuric acid complete the instantaneous acidolysis reaction through the pulse jet reactor on their respective pipelines. The concentration of concentrated sulfuric acid is 94%, the pH value is controlled at 1.7, and the reaction temperature is 82°C;
[0028] 2. Add calcium hydrogen citrate and sulfuric acid to the crystallization tank for the acid solution to fine-tune the pH value and complete the crystallization process. The pH value is controlled at 3.0, and the crystallization temperature is 85°C;
[0029] 3. The crystal slurry liquid is classified into calcium sulfate particles in the crystal growth tank, and the fine particles return to the crystallization tank with a reflux ratio of 0.5. The coarse particles of 80-90 mesh enter the filtration section to complete the solid-liquid separation, and the acidity of the obtained acid hydrolysis solution is 50.42%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com