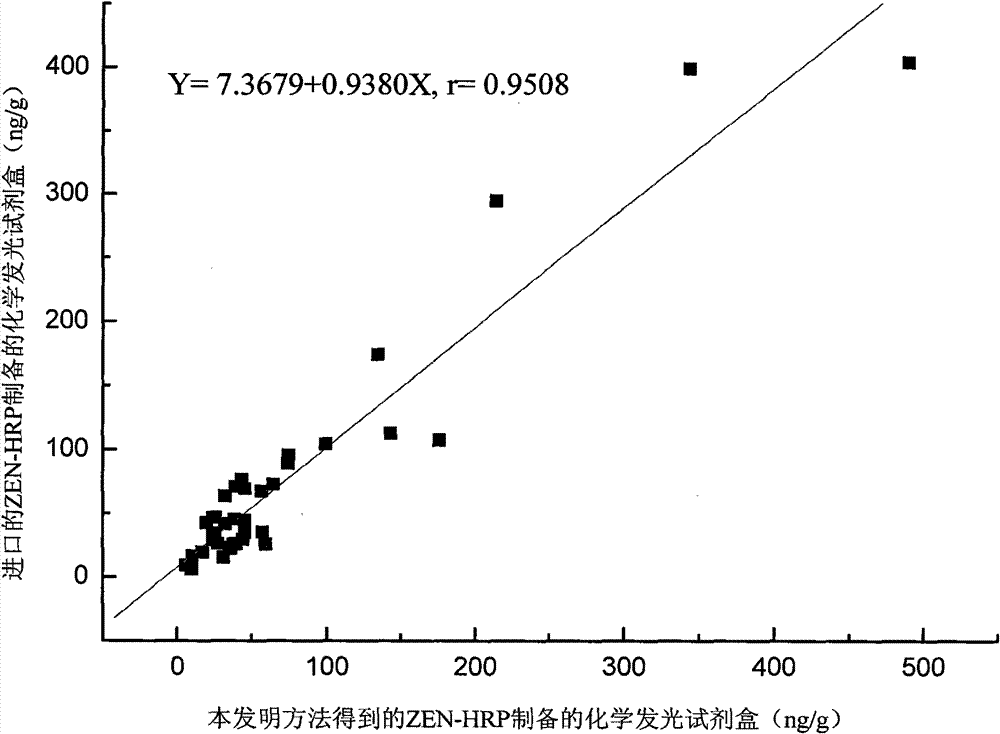
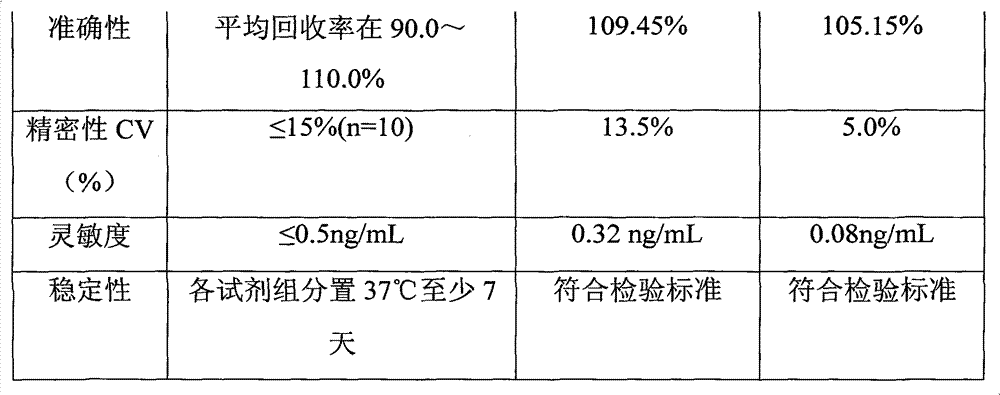
Synthesis process of horseradish peroxidase enzymelabeled zearalenone
A technology of horseradish peroxidase and zearalenone is applied in the field of synthesis technology of enzyme-labeled reagents to achieve the effects of avoiding cross-linking reaction, increasing enzyme activity and improving effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of horseradish peroxidase-labeled zearalenone
[0028] Weigh 0.5mg ZEN and 4.0mg carboxymethylhydroxylamine hydrochloride, dissolve in 0.2ml pyridine, and stir at room temperature for 24h. Add 0.2ml of distilled water to the reaction solution, adjust the pH to 8.5 with 0.2M NaOH, and shake to clarify the solution. Extracted 3 times with benzene (0.2ml), adjusted the pH of the aqueous layer to 3 with 2M HCl, extracted 3 times with ethyl acetate (0.5ml), and dried in vacuo, the obtained carboxylated product was carboxylated zearalenone .
[0029] Weigh 0.2 mg carboxylated zearalenone, 0.4 mg succinimide and 1 mg carbodiimide, dissolve in 0.7 ml DMF, and stir at room temperature for 1 h. Slowly add this reaction solution dropwise to 0.1M NaHCO containing 13.6mg HRP 3 The solution was stirred at room temperature for 2 hours. Dialyzed against 0.02M PBS overnight, the resulting product (ZEN-HRP) was protected with an equal volume of glycerol and store...
Embodiment 2
[0030] Example 2 Preparation of horseradish peroxidase-labeled zearalenone
[0031] Weigh 0.5mg ZEN and 4.3mg carboxymethylhydroxylamine hydrochloride, dissolve in 0.3ml dimethyl sulfoxide, and stir at room temperature for 24h. Add 0.3ml of distilled water to the reaction solution, adjust the pH to 9 with 0.2M NaOH, and shake to clarify the solution. Extracted 3 times with benzene (0.3ml), adjusted the pH of the aqueous layer to 4 with 2M HCl, extracted 3 times with ethyl acetate (0.6ml), and dried in vacuo, the obtained carboxylated product was carboxylated zearalenone .
[0032] Weigh 0.2 mg of carboxylated zearalenone, 0.4 mg of succinimide and 1 mg of carbodiimide, dissolve in 0.7 ml of ethanol, and stir at room temperature for 1 h. Slowly add this reaction solution dropwise to 0.1M NaHCO containing 6.8mg HRP 3 The solution was stirred at room temperature for 2 hours. Dialyzed against 0.02M PBS overnight, the resulting product (ZEN-HRP) was protected with an equal volum...
Embodiment 3
[0033] Example 3 Preparation of horseradish peroxidase-labeled zearalenone
[0034] Weigh 0.4mg ZEN and 3.2mg carboxymethylhydroxylamine hydrochloride, dissolve in 0.2ml pyridine, and stir at room temperature for 24h. Add 0.2ml of distilled water to the reaction solution, adjust the pH to 8 with 0.2M NaOH, and shake to clarify the solution. Extracted 3 times with benzene (0.2ml), adjusted the pH of the aqueous layer to 3 with 2M HCl, extracted 3 times with ethyl acetate (0.5ml), and dried in vacuo, the obtained carboxylated product was carboxylated zearalenone .
[0035]Weigh 0.2 mg carboxylated zearalenone, 0.4 mg succinimide and 1 mg carbodiimide, dissolve in 0.7 ml DMF, and stir at room temperature for 1 h. The reaction solution was slowly added dropwise to 0.1M NaHCO3 solution containing 10.2 mg HRP, and stirred at room temperature for 2 h. After dialyzing overnight with 0.02M PBS, the resulting product (ZEN-HRP) was protected with an equal volume of glycerol and stored...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com