Preparation method of spirofluorene acridine intermediate
A technology for spirofluorene acridine and an intermediate is applied in the field of one-pot preparation of spirofluorene acridine, and can solve the problems of complicated separation and purification of intermediate aromatic tertiary alcohols, complicated preparation, expensive raw materials of o-haloaryl reagents, etc. Promotes wide application, simple operation, and environmentally friendly results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of 10-hydro-spiro[fluorene-9,9'-acridine]
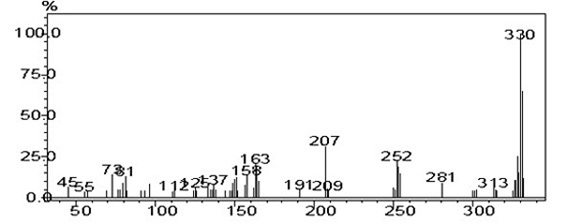
[0038] Take a dry 100 mL two-necked flask, add fluorenone (0.5031 g, 2.79 mmol, 1 equiv), aniline (2.20 mL, 24.1 mmol, 8.64 equiv), aniline hydrochloride (1.11 g, 14.3 mmol, 5.16 equiv) , under anhydrous and anaerobic conditions, heated to 184 ℃ and reacted for 5 hours, polyphosphoric acid (PPA) (3 mL) was added, and the reaction was continued for 20 hours. After the reaction was completed and cooled, the reaction was quenched with an appropriate amount of water, and CH 2 Cl 2 Extracted, combined the organic phases, dried, and rotary-evaporated under reduced pressure to obtain the crude product, which was purified by column chromatography using petroleum ether: dichloromethane 5:1 as the eluent to obtain a yellow powder solid 0.030 g, yield: 2%. GC-MS (EI- m / z ):331 / (M + )
Embodiment 2
[0039] Example 2: Synthesis of 14-hydro-spiro[dibenzo[c,h]acridine-7,9'-fluorene]
[0040] Take a dry 250 mL two-necked flask, add fluorenone (5.024 g, 27.9 mmol, 1 equiv), 1-naphthylamine (3.947 g, 27.6 mmol, 1 equiv), 1-naphthylamine hydrochloride (16.89 g, 94 mmol, 3.37 equiv) under anhydrous and oxygen-free conditions, heated and stirred at 180°C for 6 hours, then added polyphosphoric acid to the reaction system, and continued to react for 20 hours. After the reaction is finished, cool the reaction, add 5% aqueous sodium hydroxide solution and dichloromethane under stirring, extract, dry and rotary evaporate under reduced pressure to obtain the crude product and purify it with petroleum ether: dichloromethane 5:1 column chromatography to obtain 3.6 g of yellow powder, the yield is 30%, the purity is greater than 99%, and the melting point is higher than 300°C.
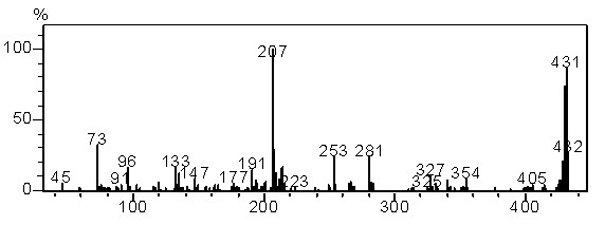
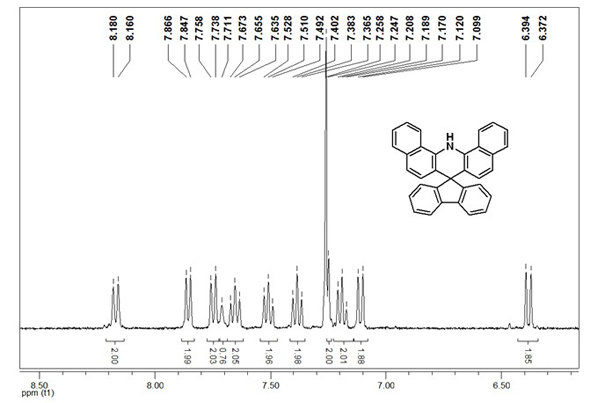
[0041] GC-MS (EI-m / z): 431 (M + ). 1 H NMR (400 MHz, CDCl 3 ): δ 8.18-8.16 (d, J = 8 Hz, 2H), 7.87-7.8...
Embodiment 3
[0044] Example 3: Synthesis of 2'-bromo-14-hydro-spiro[dibenzo[c,h]acridine-7,9'-fluorene]
[0045] Take a dry 250 mL two-necked flask, add 2-bromofluorenone (5.0 g, 19.3 mmol, 1 equiv), 1-naphthylamine (2.75 g, 19.23 mmol, 1 equiv), 1-naphthylamine hydrochloride ( 13.75 g, 76.8 mmol, 4 equiv) under anhydrous and oxygen-free conditions, heated and stirred at 180°C for 7 hours, then added polyphosphoric acid to the reaction system, and continued to react for 20 hours. After the reaction, cool the reaction, add 5% aqueous sodium hydroxide solution and dichloromethane under stirring, extract, dry and rotary evaporate under reduced pressure to obtain the crude product and purify it with petroleum ether:dichloromethane 7:1 column chromatography to obtain light yellow The powder is 1.62g, the yield is 18%, the purity is greater than 99%, and the melting point is higher than 300°C.
[0046] MALDI-TOF-MS (MALDI- m / z ): 509 / (M + ); 1 HNMR (400 MHz, CDCl 3 , ppm): δ 8.19-8.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com