Preparation method for soluble truncated human tumor necrosis factor-related apoptosis inducing ligand (TRAIL) active protein
An active protein and soluble technology, applied in the field of bioengineering, can solve the problems of unsuitable anti-tumor drugs, high cost, low yield of soluble protein, etc., and achieve the effect of simple procedure, short fermentation time and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
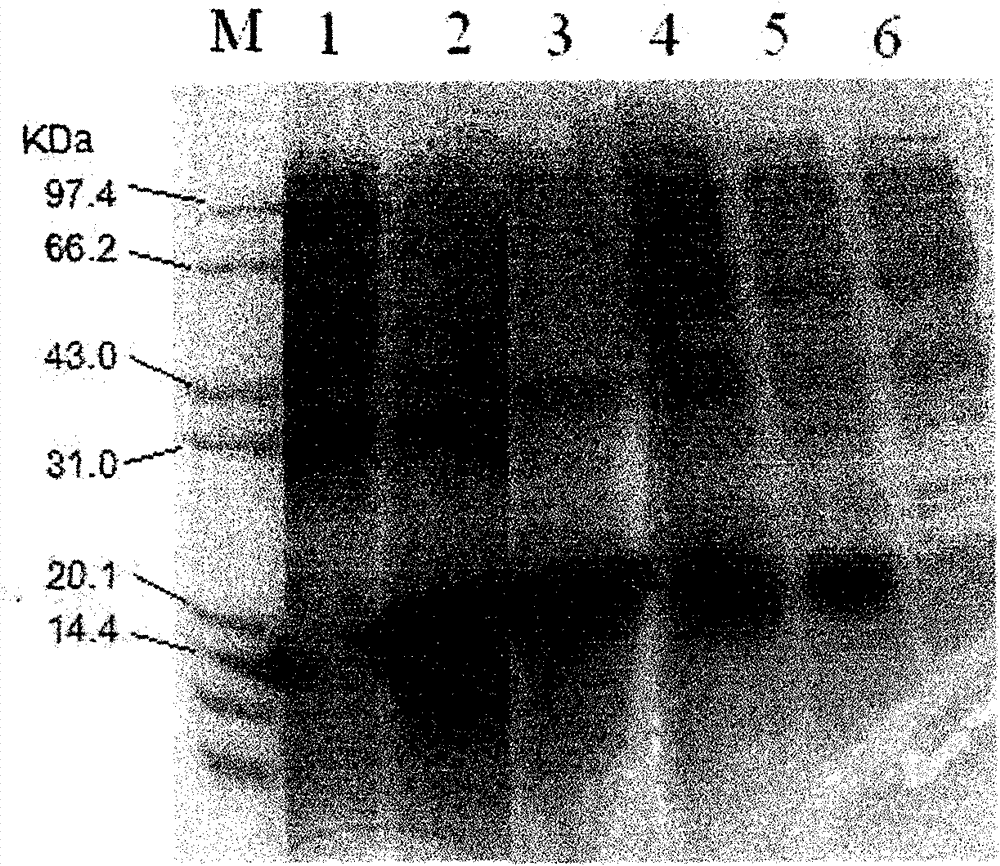
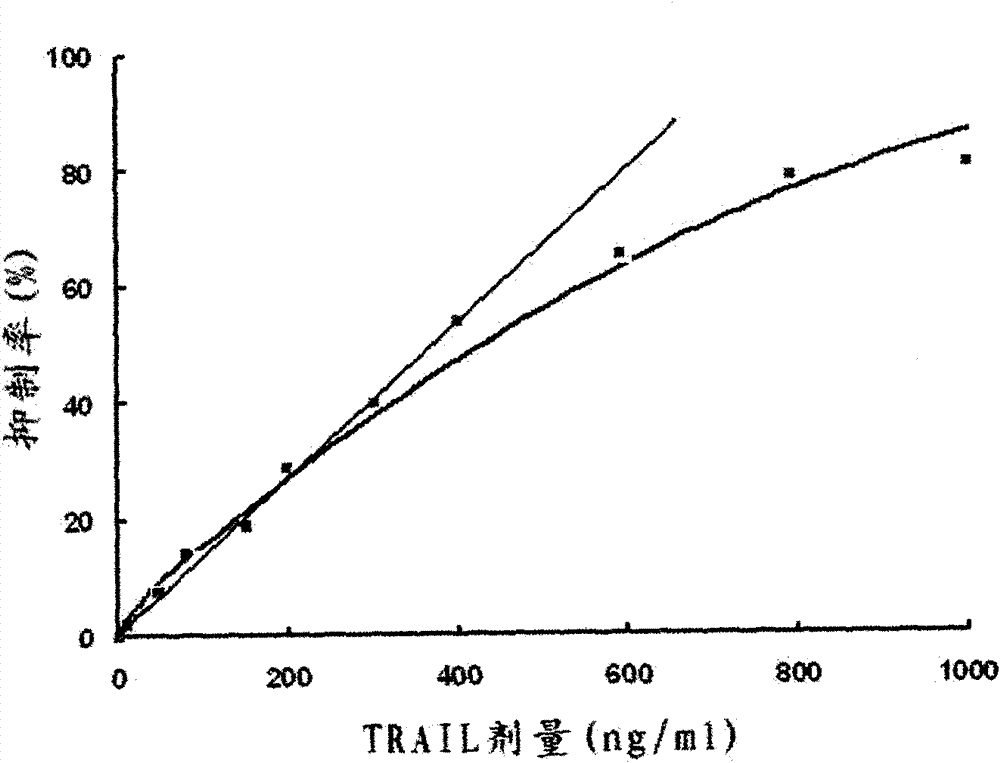
[0026] Example 1: Preparation of truncated human TRAIL protein (111-281 amino acids) fragment
[0027] First, according to the amino acid sequence of the 111-281 amino acid peptide of the human TRAIL protein published by Genbank and according to the preference of the bacterial genetic code, 24 pairs of DNA single-stranded nucleotide sequences were designed and artificially synthesized (see artificial in the nucleotide sequence list) Synthetic primers 1-24), using the above-mentioned three-round PCR method to synthesize double-stranded DNA encoding the 111-281 amino acid peptide segment of human TRAIL protein. The first round of PCR was denaturation at 94°C for 30s, 52°C for 45s, and extension at 72°C for 60s, with a total of 20 cycles of amplification; the second round of PCR was denaturation at 94°C for 5 minutes, followed by denaturation at 94°C for 30s, and 52°C for 45s. , 72°C extension for 60 s, a total of 30 cycles of amplification; the third round of PCR was denaturatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com