Alpha spiral cation polypeptide molecule and preparation method and application thereof
A cationic polypeptide and helical technology, which is applied to alpha helical cationic polypeptide molecules and their preparation methods and application fields, to achieve the effects of high charge density, high gene expression efficiency and mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The general molecular formula of the polypeptide in claim 1 is taken as n1=3, n2=1, x=1, y=1, z=0, B1 is Ala, and B2 is Lys. The structure of the polypeptide molecule is:
[0055]
[0056] Abbreviated as RC16. This example illustrates the synthesis of RC16 and its use in gene delivery. RC16 was synthesized by a peptide synthesizer and purified by HPLC.
[0057] Dissolve 10mg of RC16 in 20mM PBS buffer (pH 5.0), mix the RC16 solution with DNA according to a certain nitrogen-phosphorus ratio (N / P), and the N / P value is 0, 1, 5, 10, 20 , 30, 40 and 50. The mixture was left at room temperature for 30 minutes to fully combine RC16 with DNA to form a stable RC16 / DNA complex. The complex had an average particle diameter of 540 nm and a zeta potential of 22 mV.
[0058] HepG2 cells were seeded in 24-well plates (8×10 4 cells / well), put into an incubator, and when the confluence of the cells reaches 70%, remove the medium, rinse the cells twice with PBS buffer, and then...
Embodiment 2
[0060] The molecular general formula of the polypeptide in claim 1 is set as n1=5, n2=4, x=7, y=12, z=5, B1 is His, and B2 is Arg. n2=4 means that the molecule is a tetra-crosslinked polypeptide, referred to as tetra-RC49, and the corresponding monomer molecule is referred to as RC49.
[0061] Its structural formula is:
[0062]
[0063] This example illustrates the synthesis of tetra-RC49 and its use in gene delivery. The structural formula of tetra-RC49 is:
[0064]
[0065] Firstly, RC49 was synthesized by a peptide synthesizer, and purified by preparative liquid chromatography to obtain a monomeric polypeptide molecule.
[0066] Dissolve 10 mg of purified RC49 in deionized water to a concentration of 10 mg / ml; dissolve 400 mg of azodicarbonamide (N, N, N', N'-tetramethylazodicarboxamide) in DMSO to a concentration of 0.1 mg / μL. Then the two solutions were mixed so that the molar ratio of RC49 and azodicarbonamide was about 1:100. After stirring at room temperatu...
Embodiment 3
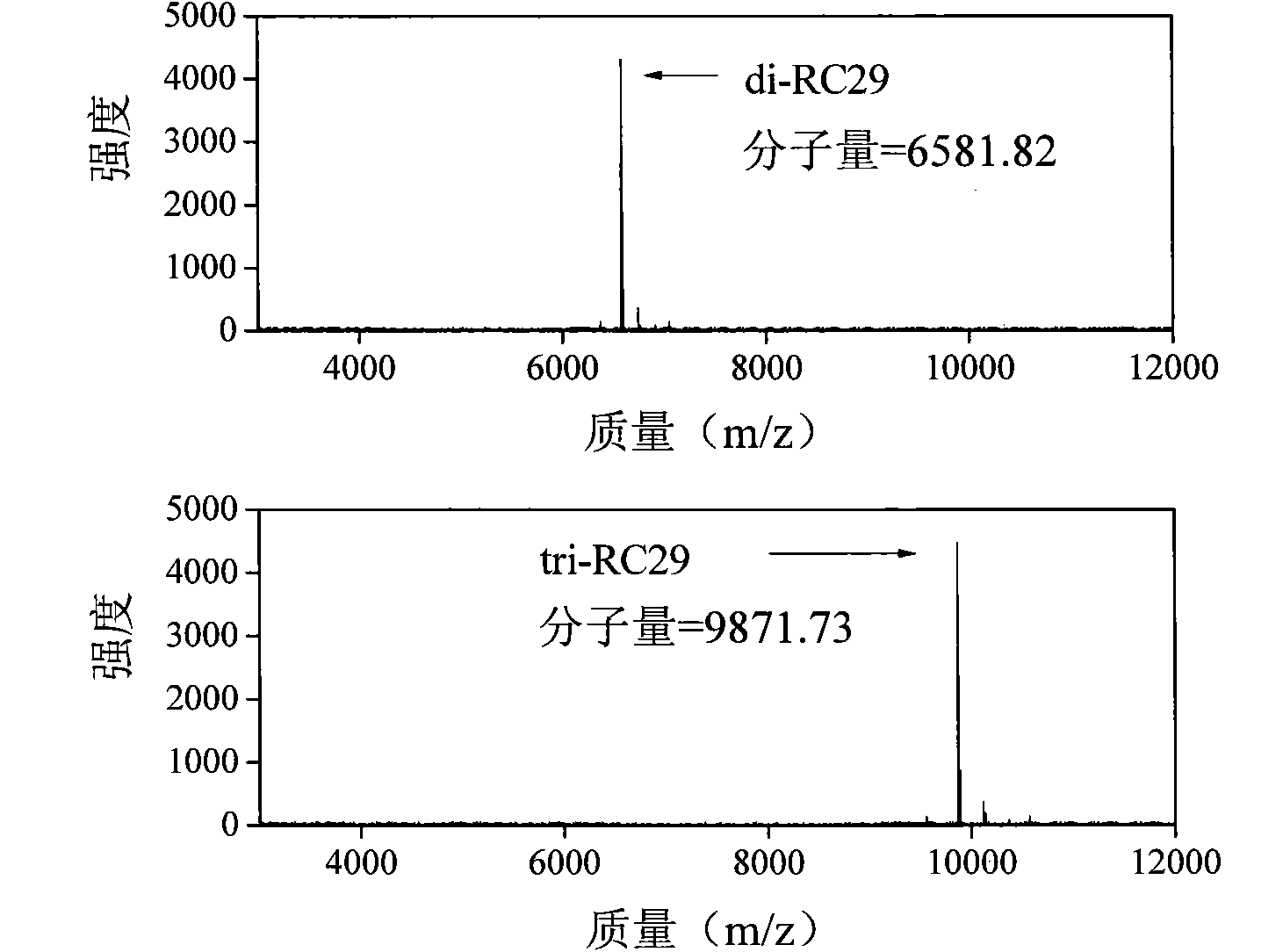
[0070] In claim 1, the molecular general formula of the polypeptide takes n1=4, n2=2, x=2, y=4, z=3, B1 is His, and B2 is Lys. n2=2 means that the molecule is a di-crosslinked polypeptide, referred to as di-RC30, and the corresponding monomer molecule is referred to as RC30, and its structural formula is:
[0071]
[0072] This example illustrates the synthesis of di-RC30 and its use in gene delivery. The structural formula of di-RC30 is:
[0073]
[0074] Firstly, RC30 was synthesized by a peptide synthesizer and purified by HPLC.
[0075] 10 mg of purified RC30 was dissolved in deionized water at a concentration of 50 mg / ml; 24 mg of azodicarbonamide (N, N, N', N'-tetramethylazodicarboxamide) was dissolved in DMSO at a concentration of 2 mg / μL. Then the two solutions were mixed so that the molar ratio of RC30 to azodicarbonamide was about 1:52. After stirring at room temperature for 3 days, the by-products were removed by dialysis with deionized water (MWCO=500Da). A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com