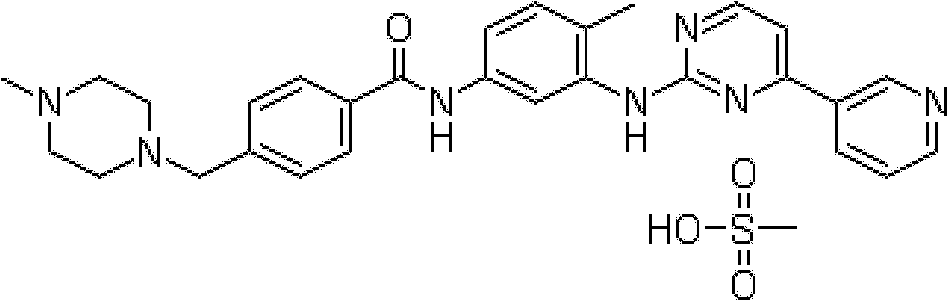
Inclusion compound of alpha crystal imatinib and preparation method thereof
A technology of inclusion complex and crystal form, which is applied in the field of inclusion complex of α crystal form imatinib and its preparation, can solve the problem that there is no reported preparation method of stable α crystal form imatinib, etc. Effects of stability and ease of operation, good fluidity, and low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Preparation of liquid inclusion compound of α-crystalline imatinib β-cyclodextrin
[0020] Weigh 0.1 g of α-crystalline imatinib and add 5 ml of absolute ethanol to dissolve it. Then weigh 19.9 g of β-cyclodextrin and use 500 ml of water to make a saturated solution in a constant temperature water bath at 50° C., slowly add the ethanol solution of α crystal form imatinib dropwise under stirring (or ultrasonic) at 50 rpm, and continue stirring ( or ultrasound) for 10 minutes, stop heating, continue stirring, that is.
[0021] It is said that the β-cyclodextrin ring can use cyclodextrin or its derivatives such as hydroxypropyl-β-cyclodextrin or dimethyl-β-cyclodextrin or dihydroxypropyl--β-cyclodextrin Alcohol or maltotriose cyclodextrin or maltotriose cyclodextrin or β-cyclodextrin sulfobutyl ether or hydroxyethyl--β-cyclodextrin or methyl-β-cyclodextrin or glucosyl-β-cyclodextrin One of the dextrins or a mixture of several of them can be used instead.
[0...
Embodiment 2
[0023] Example 2: Preparation of α-crystalline imatinib hydroxypropyl-β-cyclodextrin solid inclusion compound
[0024] Weigh 3 g of α crystal form imatinib and add 5 ml of absolute ethanol to dissolve it. Then weigh 97g of hydroxypropyl-β-cyclodextrin and use 500ml of water to make a saturated solution in a constant temperature water bath at 70°C, and slowly add the ethanol solution of α-crystalline imatinib dropwise under 100rpm stirring (or ultrasonic), Continue to stir (or sonicate) for 30 minutes, stop heating, continue to stir, refrigerate for 24 hours, filter with suction, wash, freeze-dry the precipitate, and obtain it.
Embodiment 3
[0025] Example 3: Preparation of α-crystalline imatinib hydroxypropyl-β-cyclodextrin solid inclusion complex by grinding method
[0026] Put 6 g of α crystal form imatinib and 194 g of cyclodextrin or its derivatives in a colloid mill or ball mill or mortar, add an appropriate amount of solvent to make a paste, grind for 1-3 hours, and place in Dry at a temperature of 40-70°C to obtain a solid clathrate of α-crystalline imatinib.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com