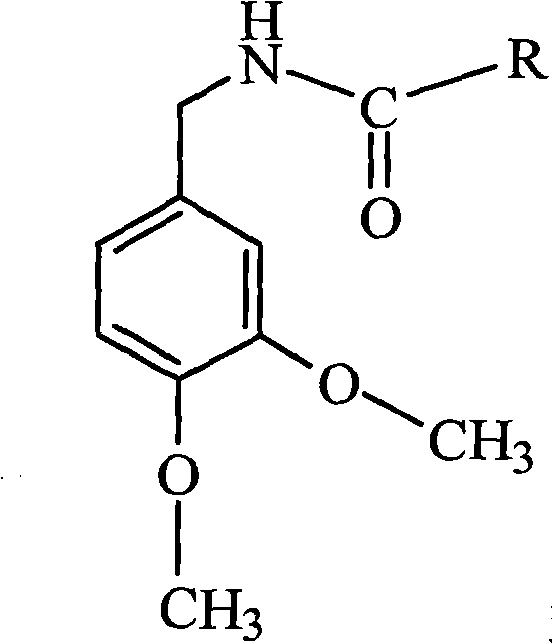
Method for synthesizing N-(3,4-dimethoxybenzyl)amide capsaicine homologous compounds
A technology of dimethoxybenzyl and capsaicin, which is applied in the field of chemical synthesis of capsaicin homologues, can solve problems such as high prices, and achieve the effects of easy-to-obtain raw materials, simple process, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Synthesis of vanillin aldoxime
[0036] Dissolve 7.6g of vanillin in 50mL of absolute ethanol and heat to 70°C, then add dropwise an aqueous solution of 4.2g of hydroxylamine hydrochloride and 2.4g of sodium hydroxide. After the dropwise addition was completed, the reaction was incubated at 70° C. for 3 h. Cool to room temperature, filter, and distill the filtrate to remove the solvent under reduced pressure. Then 50 mL of ether was added, and the ether phase was washed with deionized water. The aqueous phase was removed, and the ether phase was dried over anhydrous sodium sulfate. Filter to obtain anhydrous ether solution of vanillin aldoxime.
[0037] (2) Synthesis of vanillylamine hydrochloride
[0038] Add 5.6g of lithium aluminum hydride and about 300mL of anhydrous ether into a 500mL three-neck flask, then add 4-hydroxy-3-methoxybenzaldehyde oxime anhydrous ether solution dropwise, and react at room temperature for 12 hours after the addition is complete. ...
Embodiment 2
[0045] (1) Synthesis of vanillin aldoxime
[0046] Dissolve 15.2g of vanillin in 80mL of absolute ethanol and heat to 70°C, then add dropwise an aqueous solution of 8.0g of hydroxylamine hydrochloride and 4.5g of sodium hydroxide. After the dropwise addition was completed, the reaction was incubated at 70° C. for 3 h. Cool to room temperature, filter, and distill the filtrate to remove the solvent under reduced pressure. Then 60 mL of ether was added, and the ether phase was washed with deionized water. The aqueous phase was removed, and the ether phase was dried over anhydrous sodium sulfate. After filtration, the filtrate was distilled off under reduced pressure to remove ether, and 14.8 g of vanillin aldoxime solid powder was obtained, with a yield of 89%.
[0047] (2) Synthesis of vanillylamine hydrochloride
[0048] In a 250mL three-necked reaction flask, add 8.4g of 4-hydroxy-3-methoxybenzaldehyde oxime, 150mL of absolute ethanol, 15mL of concentrated hydrochloric ac...
Embodiment 3
[0055] (1) Synthesis of 3,4-dimethoxybenzaldehyde
[0056] Add 16.0 g of vanillin, 12.2 g of tetramethylammonium bromide, 10.5 g of potassium carbonate, and 100 mL of dimethyl carbonate into a 250 mL three-necked flask, and react under reflux for 8 hours. Cool down to room temperature, filter, adjust the pH of the filtrate to 1-2 with dilute hydrochloric acid, and then extract with methyl tert-butyl ether for 3 times. The extracts were combined, washed successively with 1N NaOH solution and saturated brine, and dried over anhydrous sodium sulfate. Filter and distill off methyl tert-butyl ether under reduced pressure to obtain 4.2 g of 3,4-dimethoxybenzaldehyde with a yield of 24%.
[0057] (2) Synthesis of 3,4-dimethoxybenzaldehyde oxime
[0058] Dissolve 4.2g of 3,4-dimethoxybenzaldehyde in 25mL of absolute ethanol and heat to 70°C, then add dropwise an aqueous solution of 2.1g of hydroxylamine hydrochloride and 1.2g of sodium hydroxide. After the dropwise addition was com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com