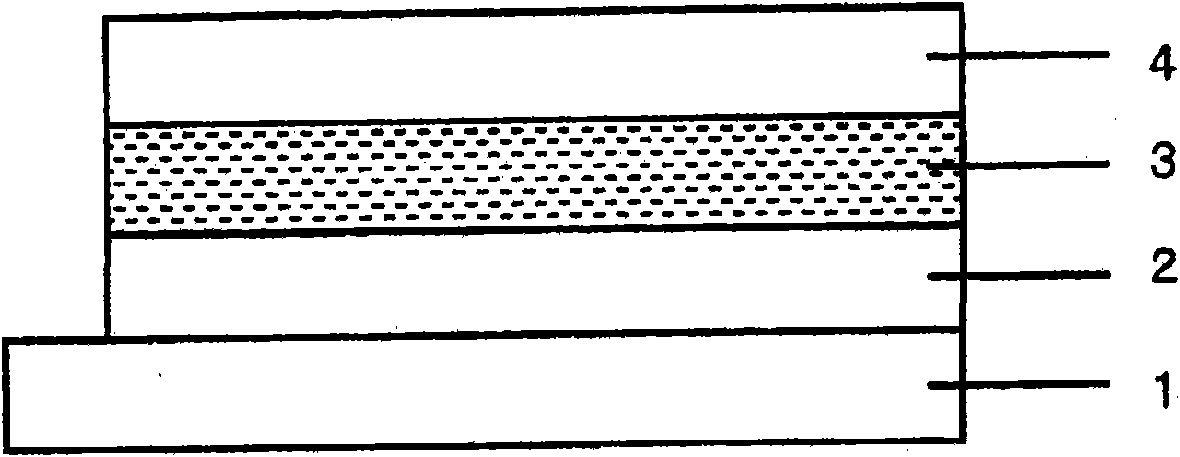
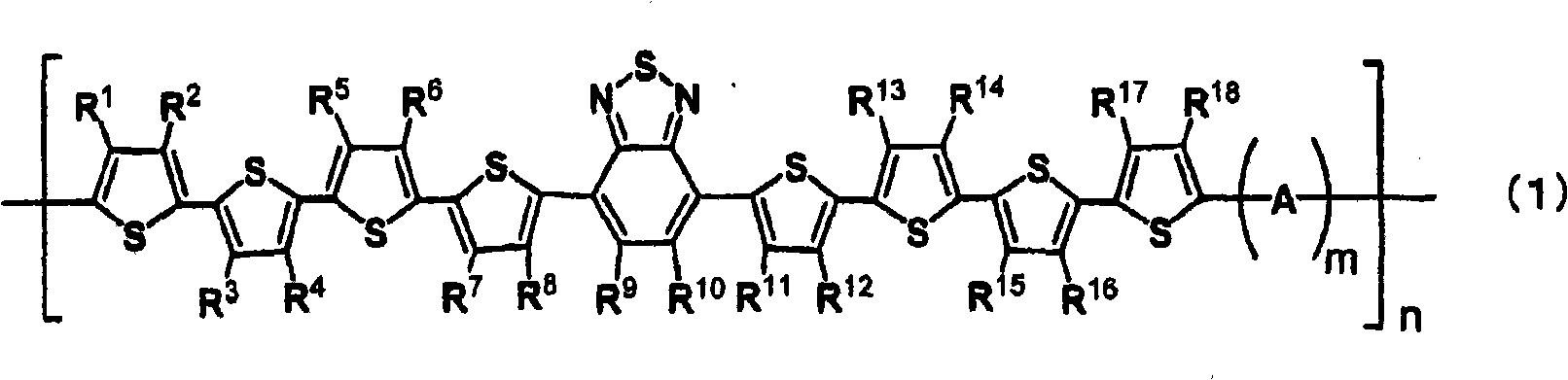
Electron donating organic material, material for photovoltaic element, and photovoltaic element
An organic material and electron-donating technology, applied in the field of photovoltaic elements, can solve the problems of low photoelectric conversion efficiency and achieve high photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0102] Hereinafter, based on an Example, this invention is demonstrated more concretely. In addition, this invention is not limited by the following Example. In addition, among the compounds used in the Examples and the like, the compounds using the abbreviations are as follows.
[0104] PEDOT: Polyethylenedioxythiophene
[0105] PSS: polystyrene sulfonate
[0106] PC 70 BM: Phenyl C71 methyl butyrate
[0108] Eg: band gap
[0109] HOMO: Highest Occupied Molecular Orbital
[0110] Isc: short circuit current density
[0111] Voc: open circuit voltage
[0112] FF: fill factor
[0113] η: Photoelectric conversion efficiency
[0114] and, 1 An FT-NMR apparatus (JEOLJNM-EX270 manufactured by JEOL Ltd.) was used for the H-NMR measurement. In addition, the average molecular weight (number average molecular weight, weight average molecular weight) was calculated by the absolute calibration curve method using a ...
Synthetic example 1
[0119] Compound A-1 is synthesized by the method shown in formula 1.
[0120] [chemical 12]
[0121]
[0122]Add 4.3 g of compound (1-a) (manufactured by Tokyo Chemical Industry Co., Ltd.) and 10 g of bromine (manufactured by Wako Pure Chemical Industries Ltd.) to 150 ml of 48% hydrobromic acid (manufactured by Wako Pure Chemical Industries Ltd.) , stirred at 120°C for 3 hours. After cooling to room temperature, the precipitated solid was filtered with a glass filter and washed with 1000 ml of water and 100 ml of acetone. The obtained solid was vacuum-dried at 60° C. to obtain 6.72 g of compound (1-b).
[0123] 10.2 g of compound (1-c) (manufactured by Tokyo Chemical Industry Co., Ltd.) was dissolved in 100 ml of dimethylformamide (manufactured by Kishida Chemical Co., Ltd.), and N-bromosuccinimide (manufactured by Co., Ltd. Wako Junyaku Kogyo) 9.24 g was stirred at room temperature for 3 hours under a nitrogen atmosphere. To the obtained solution were added 200 ml of w...
Synthetic example 2
[0131] Compound A-2 is synthesized by the method shown in formula 2.
[0132] [chemical 13]
[0133]
[0134] 0.734 g of the above-mentioned compound A-1 was dissolved in 15 ml of chloroform (manufactured by Nakarai Tesque), and 0.23 g of N-bromosuccinimide (manufactured by Tokyo Chemical Industry Co., Ltd.) / dimethylformamide (( Co., Ltd., Wako Pure Chemical Industries, Ltd.) 10 ml was stirred under a nitrogen atmosphere at room temperature for 9 hours. To the obtained solution were added 100 ml of water and 100 ml of chloroform, and the organic layer was separated, washed with 200 ml of water, and dried over magnesium sulfate. The obtained solution was purified by column chromatography (filler: silica gel, eluent: dichloromethane / hexane) to obtain 0.58 g of compound (2-a).
[0135] 0.5 g of compound (2-b) (manufactured by Tokyo Chemical Industry Co., Ltd.), 0.85 g of bis(pinacolato) diboron (manufactured by BASF), potassium acetate (manufactured by Wako Pure Chemical Ind...
PUM
| Property | Measurement | Unit |
|---|---|---|
| band gap | aaaaa | aaaaa |
| energy level | aaaaa | aaaaa |
| energy level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com