Application of lariciresinol to preparing medicament for resisting autoimmune disease and graft rejection disease
A technology for larch resin and autoimmune diseases, which is applied in metabolic diseases, skin diseases, bone diseases, etc., can solve the problems of cell non-specificity, high price, and increase the economic burden of patients, and achieve inhibition of occurrence and development, rich sources, The effect of saving treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 larch resin alcohol
[0037] Grind 50Kg of the dried bark of Daphne velvet, reflux extract with 400L of 80% ethanol for 3 times, each time for 3 hours, combine the extracts, concentrate the extracts under reduced pressure to form a liquid extract, add 50L of water to dilute, and then extract with ethyl acetate. 50 L each time, a total of 3 times to obtain 1600 g of ethyl acetate. Apply silica gel column chromatography to the above-mentioned ethyl acetate part, and use the petroleum ether / ethyl acetate system gradient elution with a volume ratio of 100:0 to 1:1, and detect by thin layer chromatography, collect the fraction containing larch resin alcohol, Then, through Sephadex gel chromatography and eluting with chloroform / methanol with a volume ratio of 1:1, 2.15 g of pure larchoresinol compound was obtained.
Embodiment 2
[0038] Example 2 In vitro culture of human dendritic cells and treatment of DCs with agaricol
[0039] Take 50ml / person of peripheral blood from healthy adults intravenously, anticoagulate with heparin, separate peripheral blood mononuclear cells by density gradient centrifugation, suspend cells in RPMI1640 (1640 medium) containing 10% calf serum, and adjust the cell concentration to 2× 10 6 / ml, add to 24-well culture plate, 0.5ml / well, incubate at 37°C, 5% CO2 for 2 hours, make the mononuclear cells adhere to the wall, wash the culture plate lightly with warm serum-free RPMI1640 to remove the adherence cells, that is, to obtain adherent monocytes. Add serum-containing RPMI 1640 containing rhGM-SCF (human recombinant granulocyte colony-stimulating factor) 1000IU / ml and rhIL-4 (human recombinant interleukin 4) 800IU / ml to the culture plate, at 37°C, 5% CO2 After culturing in an incubator for 5 days, add larch resin alcohol with a final concentration of 4 μmol / L for 24 hours,...
Embodiment 3
[0041] Example 3 Effect of larchoresinol on secretion of IL-10 by human dendritic cells
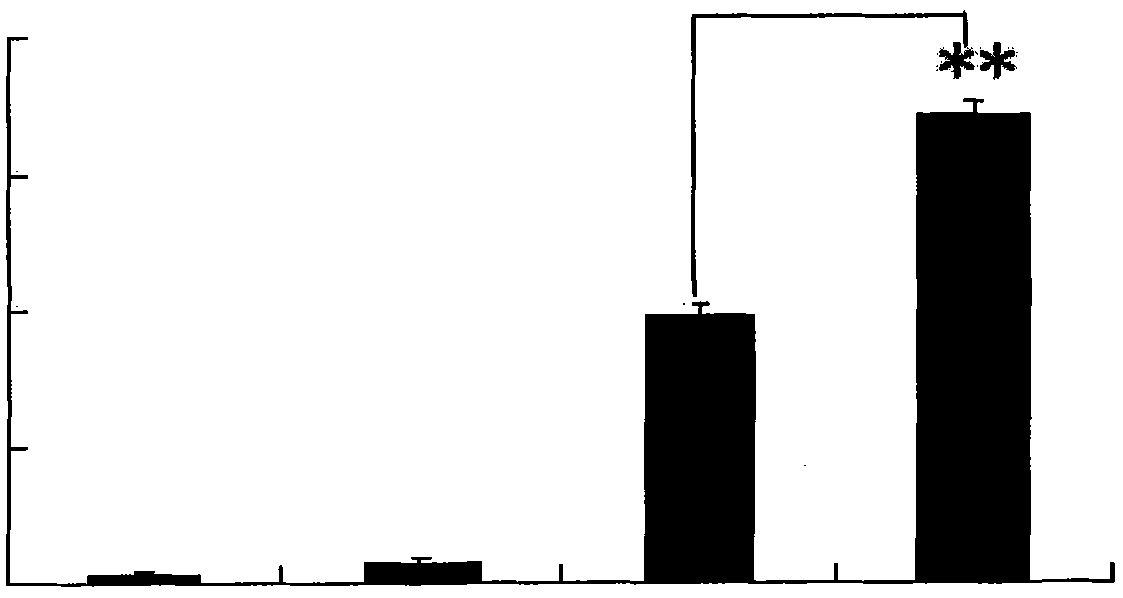
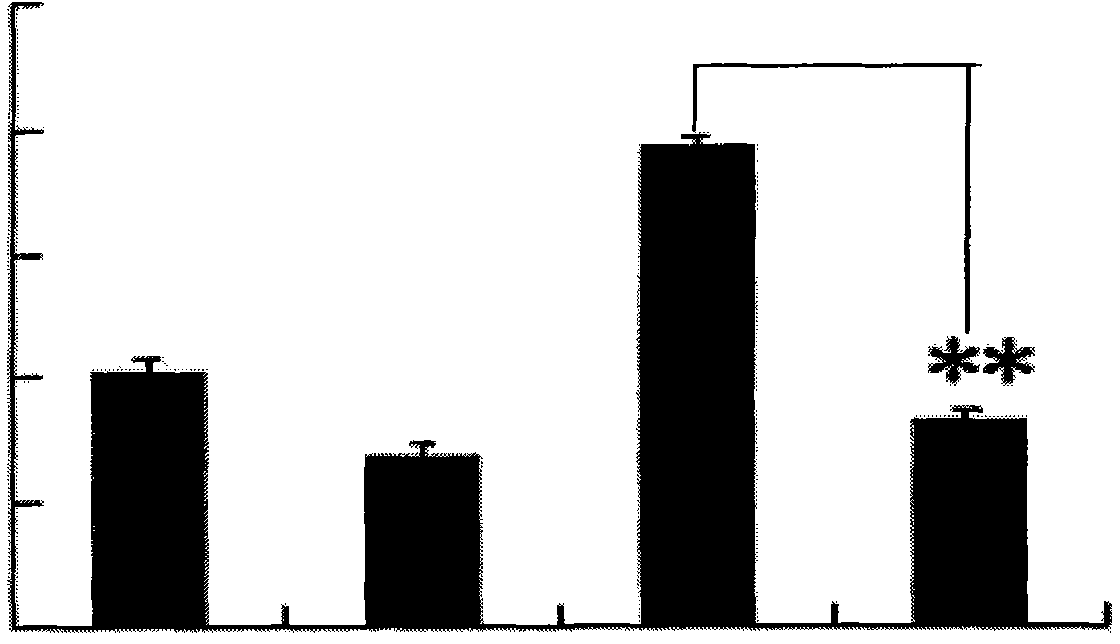
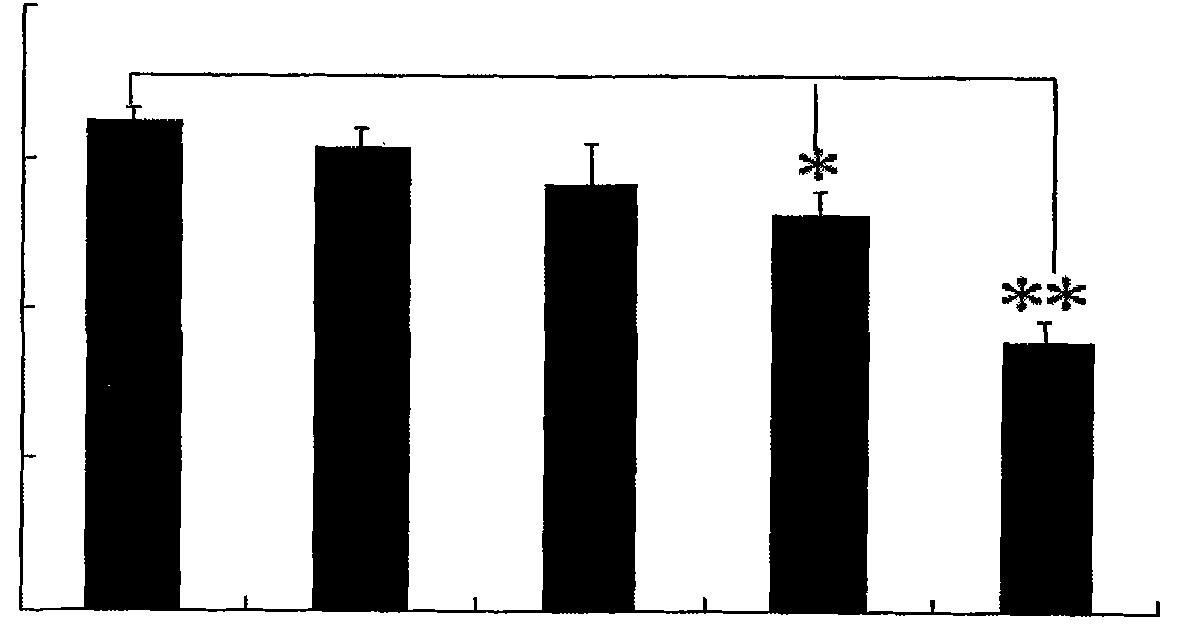
[0042] The dendritic cells of the agarisol-stimulated group and the non-stimulated group were cultured according to the method in Example 1, the supernatant was collected, and the IL-10 content was detected with an IL-10 ELISA (enzyme-linked immunosorbent assay) kit. The concentration of IL-10 secreted by mononuclear cells transformed dendritic cells in vitro in the agarisol stimulation group and the unstimulated group was 2000 ± 22pg / ml, 1200 ± 14pg / ml, and there was a significant difference (Pfigure 1 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com