Redox shuttles for high voltage cathodes
An electrochemical and battery technology, applied in battery electrodes, electrochemical generators, circuits, etc., can solve problems such as increasing cost and complexity, and achieve the effects of good capacity retention, high average voltage, and high initial capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0074] Octafluoronaphthalene was purchased from Aldrich Chemical and used as received. 2-Methoxyheptafluoronaphthalene was synthesized as described in the following preparation example. Mesophase carbon microspheres (MCMB) used to make anodes were purchased from E-One / Moli Energy Canada (Maple Ridge, B.C., Canada). LiFePO for making cathode 4 Available from Phostech Lithium (Quebec, Canada). Ethylene carbonate (EC), propylene carbonate (PC) and dimethyl carbonate (DMC) were purchased from Ferro Corp., Fine Chemicals Division (Zachary, LA), while LiPF 6 (available from Stella, Japan) was purchased from E-One / Moli Energy. Lithium bisoxalatoborate (LiBOB) was purchased from Chemetall (Germany). All solvents were high-purity battery grade and dried over 3A molecular sieves before use.
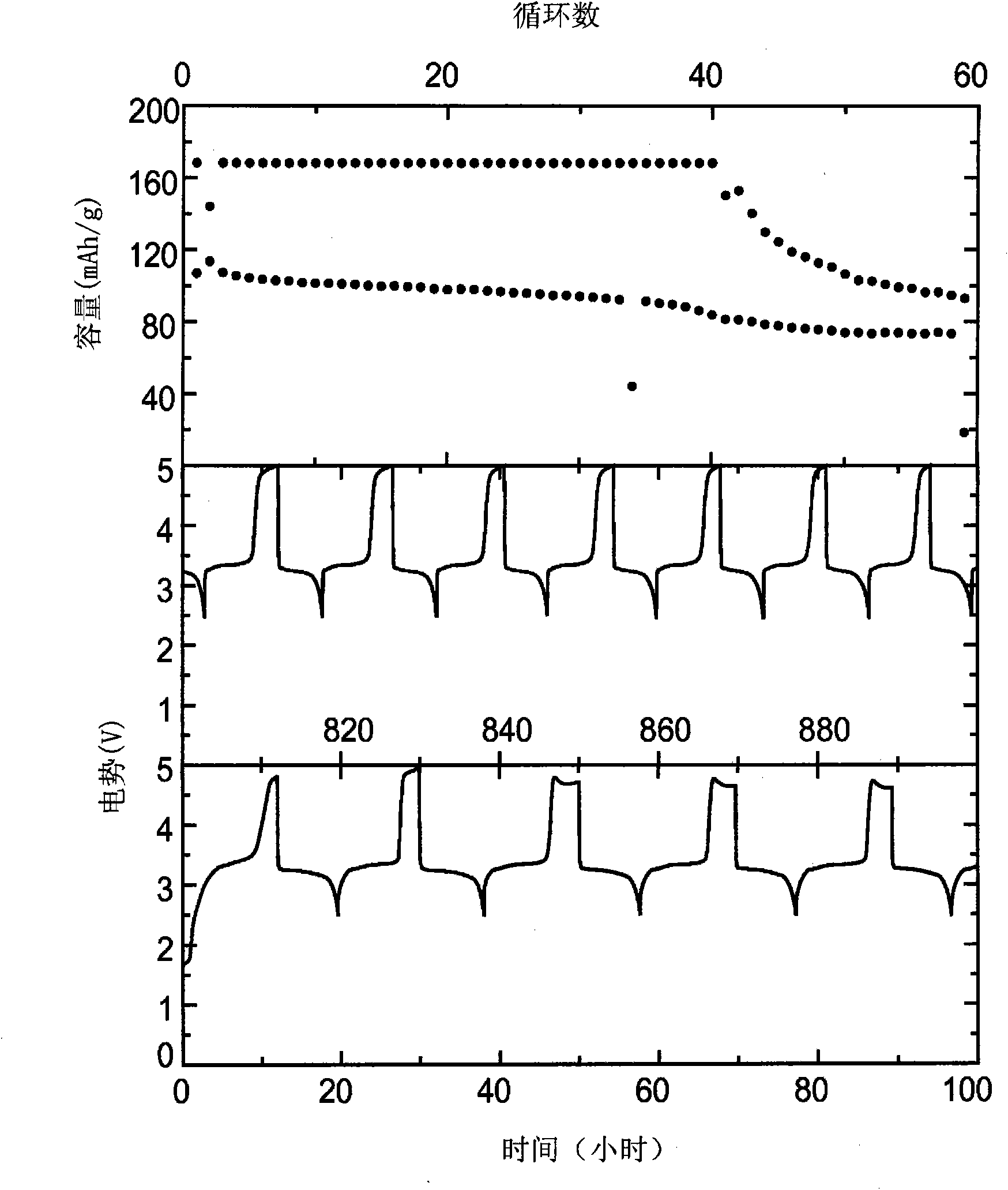
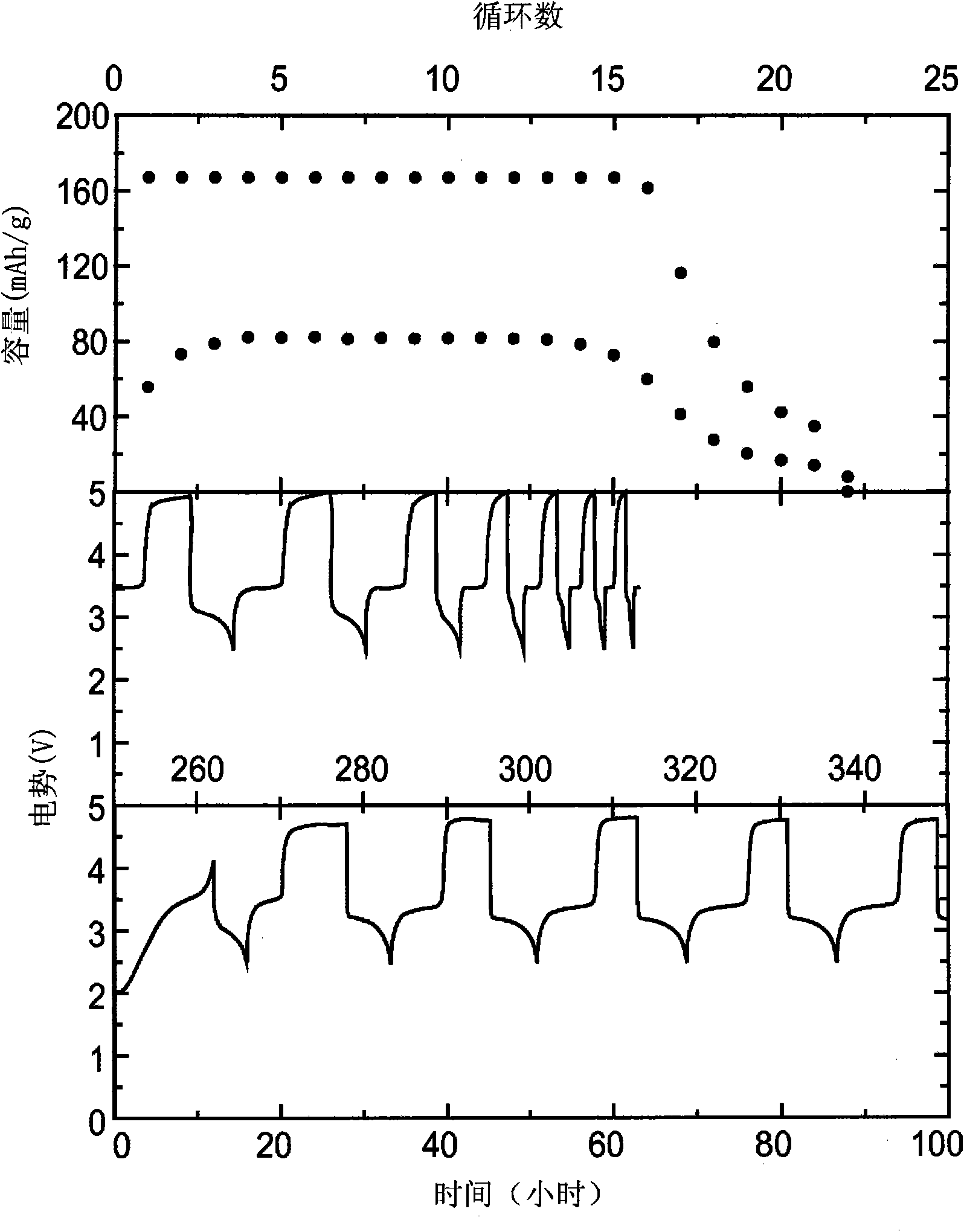
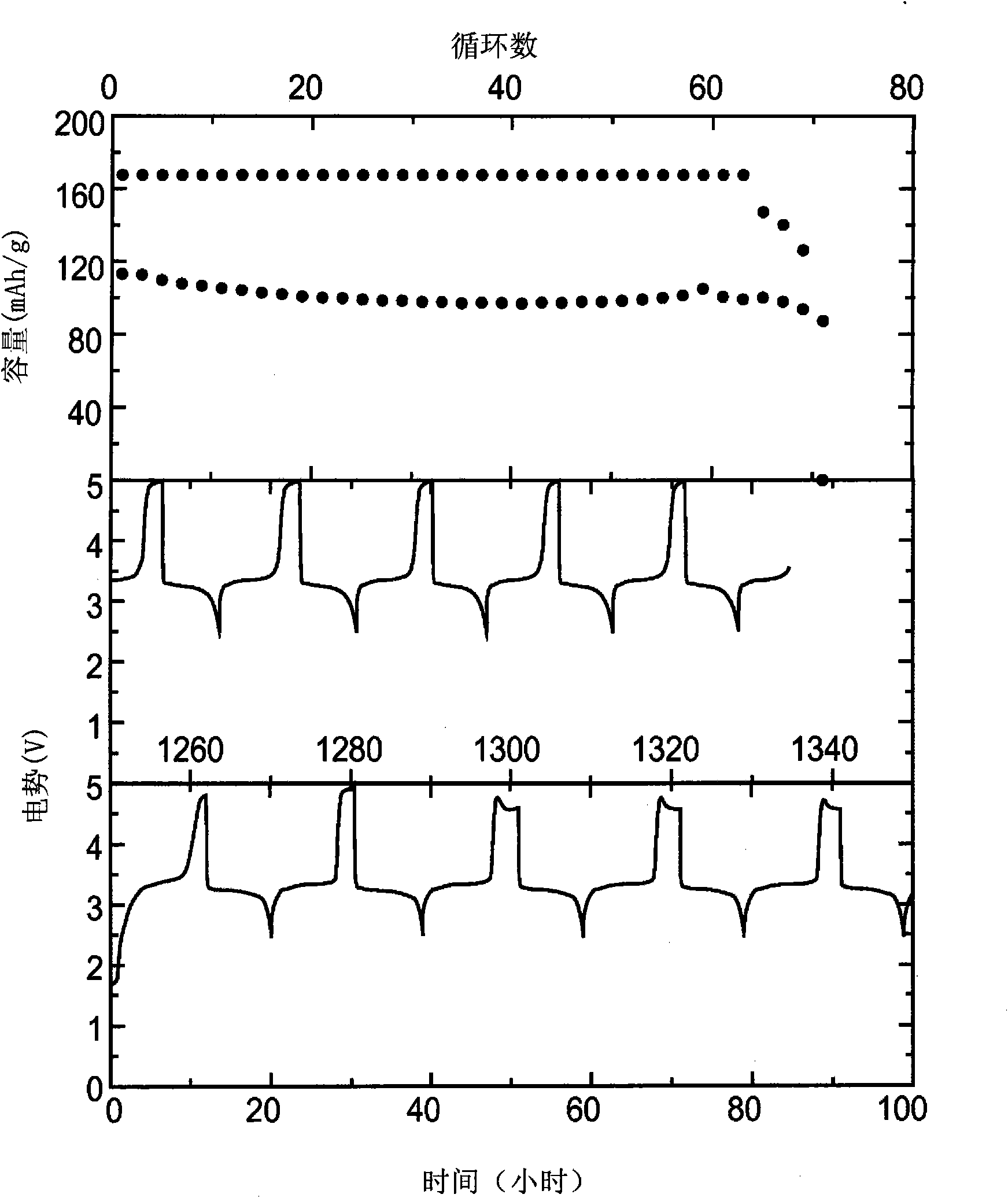
[0075] Using 0.1M chemical shuttle, 0.5M LiPF 6 Cyclic voltammograms were recorded in a standard three-electrode cell with an electrolyte composed of EC, DEC, PC, and DMC at a volume ratio of...
preparation example 1
[0079] Preparation example 1: Synthesis of 2-methoxyheptafluoronaphthalene
[0080] Octafluoronaphthalene (12 g, 0.044 mol), sodium methoxide (2.4 g, 0.0448 mol), and anhydrous methanol were combined in a 100 mL round bottom flask equipped with a reflux condenser and a dry nitrogen sparger. The mixture was refluxed for six hours. Samples were taken and analyzed by GC-FID. At six hours, there was 35% conversion to 2-methoxyheptafluoronaphthalene. An additional 2.0 g of sodium methoxide was added and the mixture was refluxed for an additional 6 hours. Additional methoxide and reaction time did not increase product conversion. The undissolved solid was then filtered at room temperature, and the crude product was recrystallized from aqueous methanol. The product was further purified by flash silica gel column chromatography using hexane as eluent. A total of 0.5 g of high-purity product was obtained. The structure was verified by GC / MS and the purity was 99.7% by GC-FID. ...
preparation example 2
[0081] Preparation Example 2: Preparation of 1,4-bis(2,2,2-trifluoroethoxy)-2,5-di-tert-butylbenzene
[0082] 2,5-di-tert-butylhydroquinone (3.23g, 0.014mol), potassium carbonate (powder, 325 mesh, 4.4g, 0.032mol), tri-n-butylamine (0.2g, 0.0011mol) and 30g The acetone solvent was mixed in a 100 mL three-necked round bottom flask. The flask was equipped with an overhead stirrer, thermocouple, addition funnel, cold water condenser, heating mantle, and dry nitrogen bubbler. Trifluoroethyl 2,2,2-perfluorobutanesulfonate (12.5 g, 0.032 mol, Aldrich) was added dropwise over a period of 2 hours while heating the reaction mixture at 58°C. After stirring for 24 hours, 80 mL of water was added at 58 °C, then the stirring was stopped and the heat source was removed. The product which crystallized in the lower phase at room temperature was filtered from the liquid using vacuum filtration. The crude product was then recrystallized in methanol / water, and the purity of the prepared sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com