Methods for synthesizing isatoic anhydride and N-isopropyl-2-aminobenzamide
A synthesis method and technology of isatoic anhydride are applied in the synthesis of N-isopropyl-2-aminobenzamide and the synthesis field of isatoic anhydride, which can solve the problem of unstable quality of bentazon preparations, increasing operation steps, prolonging Production cycle and other problems, to achieve the effect of shortening production cycle, reducing operation steps, and cleaning the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a kind of synthetic method of isatoic anhydride, comprising:
[0025] Add bis(trichloromethyl)carbonate solution dropwise to the anthranilic acid solution, and reflux reaction to obtain isatoic anhydride.
[0026] The invention uses anthranilic acid and bis(trichloromethyl)carbonate as raw materials to react in an organic solvent to generate isatoic anhydride.
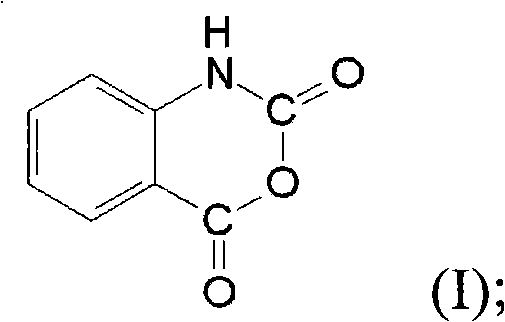
[0027] Anthranilic acid has a structure of formula (II), which is relatively similar to the structure of isatoic anhydride having a structure of formula (I).
[0028]
[0029] Bis (trichloromethyl) carbonate, also known as solid phosgene or triphosgene, the chemical formula is C 3 o 3 Cl 6 , low toxicity, relatively safe during transportation, storage and use.
[0030] According to the present invention, at first prepare anthranilic acid solution and two (trichloromethyl) carbonate solution, specifically comprise the following steps:
[0031] Anthranilic acid is mixed with an organ...
Embodiment 1
[0051] Add 20 g of commercially available anthranilic acid and 150 mL of ethylene dichloride with a purity of 98% in a 500 mL four-necked bottle equipped with a stirrer, a thermometer and a circulating condensing device, turn on the stirrer and a circulating condensing device, and heat up to 68 ℃, stirred until anthranilic acid was completely dissolved to obtain anthranilic acid solution; 16 g of commercially available bis(trichloromethyl)carbonate was dissolved in 40mL of dichloroethane to obtain bis(trichloromethyl)carbonic acid Ester solution; the bis(trichloromethyl)carbonate solution was slowly added dropwise to the anthranilic acid solution for 2 hours, then the temperature was raised to 75° C. for reflux reaction for 2 hours to obtain isatoic anhydride solution. After cooling down to room temperature, the isatoic anhydride solution was suction-filtered and weighed 22.7 g after drying. The dried product was subjected to liquid chromatography measurement, wherein the isat...
Embodiment 2
[0053] Add 20 g of commercially available anthranilic acid and 150 mL of ethylene dichloride with a purity of 98% in a 500 mL four-necked bottle equipped with a stirrer, a thermometer and a circulating condensing device, turn on the stirrer and a circulating condensing device, and heat up to 68 ℃, stirred until anthranilic acid was completely dissolved to obtain anthranilic acid solution; 16 g of commercially available bis(trichloromethyl)carbonate was dissolved in 40mL of dichloroethane to obtain bis(trichloromethyl)carbonic acid Ester solution; slowly drop the bis(trichloromethyl)carbonate solution into the anthranilic acid solution for 2 hours, then heat up to 80° C. for reflux reaction for 2 hours to obtain isatoic anhydride solution;
[0054] The temperature of the isatoic anhydride solution was lowered to 55°C, and 12 g of isopropylamine was slowly added dropwise for 2 hours, and then kept for 1 hour to obtain N-isopropyl-2-aminobenzamide solution, and the solution was su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com