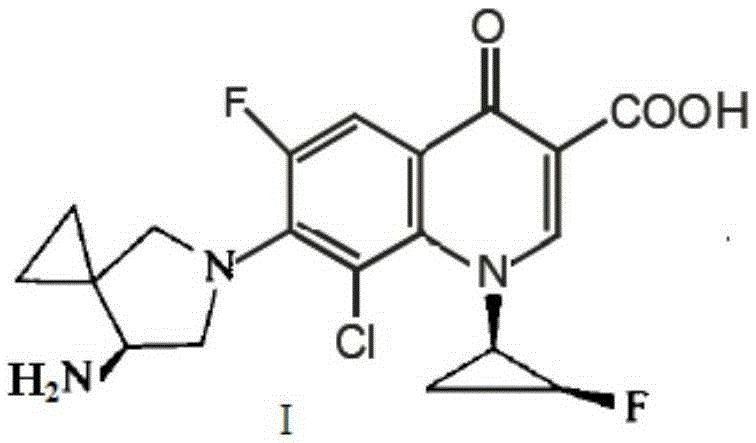
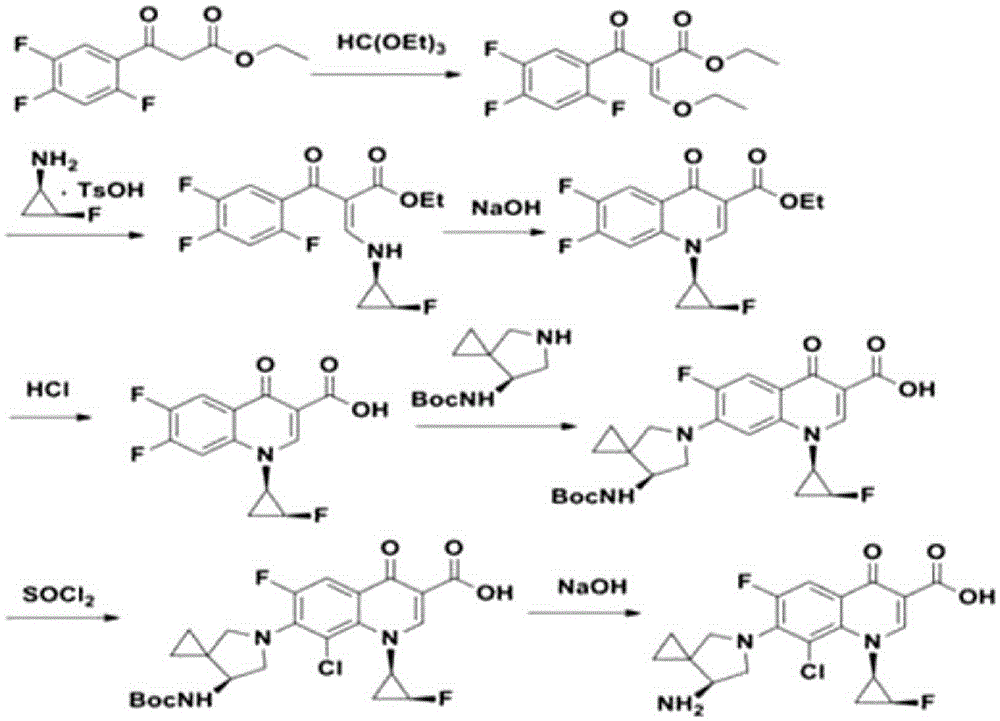
Preparation method of sitafloxacin
A technology of sitafloxacin and compounds, applied in the field of preparation of sitafloxacin, can solve the problems of low product yield, cumbersome post-processing, high cost, etc., and achieve low impurity content, high product yield and purity, and total product Effect of Yield Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of compound Ⅲ
[0034]
[0035] Under nitrogen protection, 2,3,4-trichloro-5-fluorobenzoic acid (II) (20.9g, 80mmol), (1.5g, 8mmol) cuprous iodide, (2.9g, 16mmol) 1,10-phenanthroline, 100ml N,N-dimethylformamide, (21.2g, 100mmol) anhydrous potassium phosphate, drop (39.6g, 160mmol) (1R, 2S)-(-)-cis -1-Amino-2-fluorocyclopropane p-toluenesulfonate N,N-dimethylformamide solution, reaction temperature 20-25°C, stirring reaction, maintaining reaction for 10 hours, after the reaction, adjust PH=4.5, Cooled to 0°C, filtered to obtain 21.0 g of solids, yield 93%, although the 3-position chlorine atom and 4-position chlorine atom may participate in the 2-position coupling competition reaction from theoretical analysis, the content of compound III was 99.1% through instrumental detection and analysis, The impurity A content was 0.5%, and the impurity B was not detected.
Embodiment 2
[0037] Preparation of compound Ⅲ
[0038] Under nitrogen protection, 2,3,4-trichloro-5-fluorobenzoic acid (II) (20.9g, 80mmol), (0.8g, 8mmol) cuprous chloride, (2.9g, 16mmol) 1,10-phenanthroline, 100ml N,N-dimethylformamide, (32.6g, 100mmol) anhydrous cesium carbonate, drop (29.7g, 120mmol) (1R, 2S)-(-)-cis -1-Amino-2-fluorocyclopropane p-toluenesulfonate N,N-dimethylformamide solution, reaction temperature 20-25°C, stirring reaction, maintaining reaction for 10 hours, after the reaction, adjust PH=4.5, After cooling to 0°C, 20.5 g of solid compound III was obtained by filtration with a yield of 91%. According to instrumental analysis, the content of compound III was 99.2%, the content of impurity A was 0.3%, and the content of impurity B was not detected.
Embodiment 3
[0040] Preparation of compound Ⅳ
[0041]Add (17.4g, 60mmol) compound III and 150mL dichloromethane into a 250mL four-neck flask equipped with a stirrer, thermometer and circulating condensing device, turn on the stirrer and circulating condensing device, raise the temperature to 50°C, and stir until compound III is completely Dissolved to obtain compound III solution; 20g commercially available bis(trichloromethyl)carbonate was dissolved in 40mL of dichloromethane to obtain bis(trichloromethyl)carbonate solution; the bis(trichloromethyl)carbonate ) carbonate solution was slowly added dropwise to the compound III solution for 1.5 hours, and then the temperature was raised to 60° C. for 2 hours to obtain the compound IV solution. After cooling down to room temperature, the compound IV solution was suction-filtered, dried and weighed to 18.0 g, the yield was 98%, and the HPLC purity was 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com