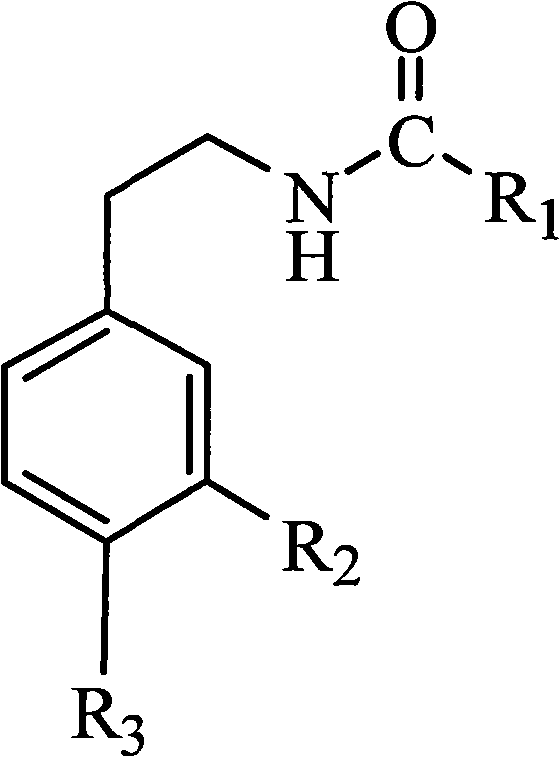
Method for synthesizing capsaicin homolog
A technology for homologues and capsaicin is applied in the field of chemical synthesis of capsaicin homologues, and achieves the effects of simple and convenient preparation process, readily available raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Synthesis of 4-hydroxy-β-nitrostyrene
[0031] Add 6.1g 4-hydroxybenzaldehyde, 3.2g ammonium acetate, 10mL nitromethane and 25mL glacial acetic acid to a 250mL single-necked flask, turn on the electromagnetic stirrer and heat, and make it reflux for 3h in an oil bath at 120~130℃ . Stop heating, cool to room temperature, pour the reaction solution into ice water, precipitate a red-yellow precipitate, and filter the filter cake with ethanol / water (ethanol / water volume ratio 2 / 5) solution recrystallized three times to obtain yellow The powder is 5.2g, and the yield is 63%.
[0032] (2) Synthesis of 4-hydroxyphenethylamine hydrochloride
[0033] Add 4.8g of lithium aluminum hydride and 300mL of anhydrous ether to a 500mL single-necked flask, add 4.9g of 4-hydroxy-β-nitrostyrene in a Sox-type drawer, and carry out the extraction and reduction reaction for 52 hours to reach the extract It is colorless. Cool to room temperature, add about 80ml of deionized water dropwise, and...
Embodiment 2
[0038] (1) Synthesis of 3,4-Dimethoxy-β-nitrostyrene
[0039] Add 16.6g of 3,4-dimethoxybenzaldehyde, 10.0g of ammonium acetate, 20mL of nitromethane and 60mL of glacial acetic acid to a 250mL single-necked flask, turn on the electromagnetic stirrer and heat it to make the oil at 120~130℃ The reaction was refluxed in the bath for 2h. Stop heating, cool to room temperature, pour the reaction solution into ice water, precipitate out, and filter, filter cake is recrystallized three times with ethanol / water (ethanol / water volume ratio is 3 / 5) solution to obtain 11.3g solid powder , The yield is 54%.
[0040] (2) Synthesis of 3,4-Dimethoxyphenethylamine hydrochloride
[0041] In a 250mL three-necked reaction flask, add 8.3g of 3,4-dimethoxy-β-nitrostyrene, 150mL of absolute ethanol, 10mL of concentrated hydrochloric acid, and 5.0g of 10% palladium on carbon. Under strong magnetic stirring , 4h hydrogenation reaction at room temperature and pressure. The catalyst was filtered off, and ...
Embodiment 3
[0046] (1) Synthesis of 4-hydroxy-3-methoxy-β-nitrostyrene
[0047] 18.6g vanillin, 9.0g ammonium acetate, 19.5mL nitromethane and 53mL glacial acetic acid were added to a 250mL single-necked flask, the electromagnetic stirrer was turned on and heated, and the reaction was refluxed in an oil bath at 120-130°C for 2h. Stop heating, cool to room temperature, pour the reaction solution into ice water, precipitate a red-yellow precipitate, and filter out. The filter cake is recrystallized three times with ethanol / water (ethanol / water volume ratio 3 / 2) solution to obtain yellow The powder is 11.1g, and the yield is 46%.
[0048] (2) Synthesis of 4-hydroxy-3-methoxyphenethylamine hydrochloride
[0049] Put 2.4g of lithium aluminum hydride and about 200mL of anhydrous ether into a 250mL single-necked flask, add 2.5g of 4-hydroxy-3-methoxy-β-nitrostyrene into the Sox-type drawer, and proceed with 60 extractions. hour. Cool to room temperature, slowly add about 40ml of deionized water, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com