Method for preparing recombinant human endostatin chitosan nanoparticles for injection
A technology of chitosan nanoparticles and vascular endothelium, applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of short half-life and poor clinical compliance, and achieve High drug loading, good biocompatibility, and uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
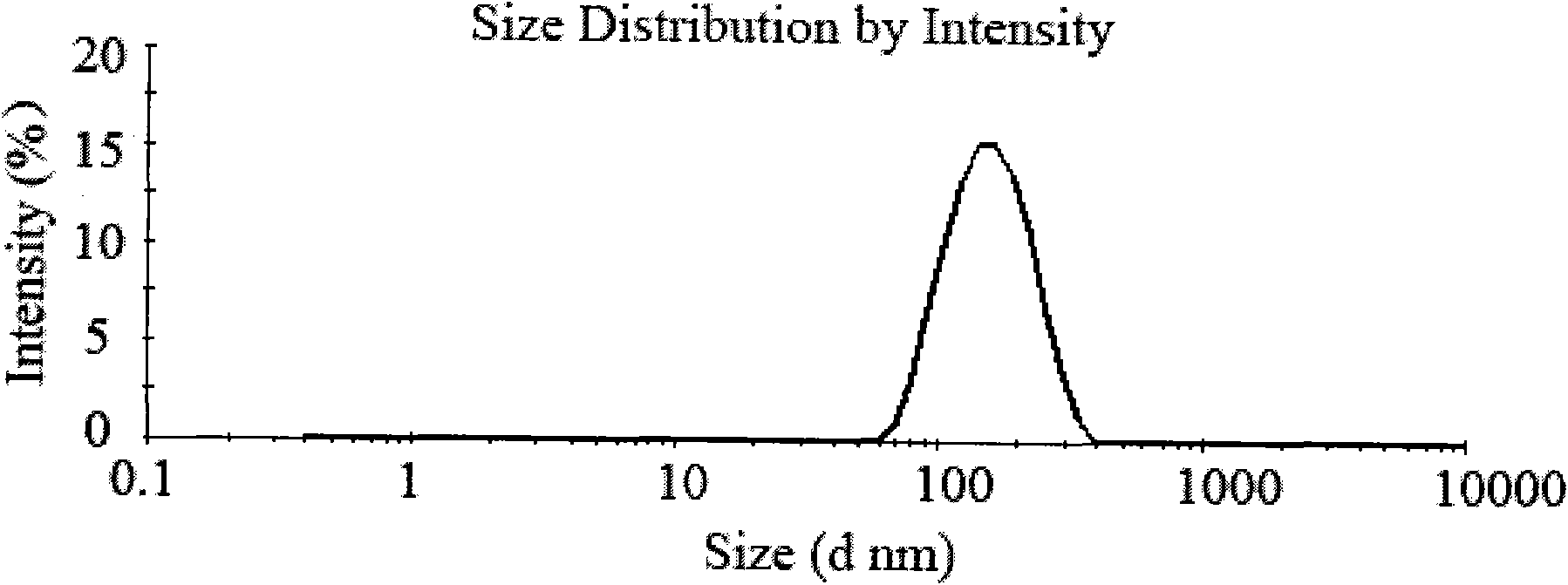
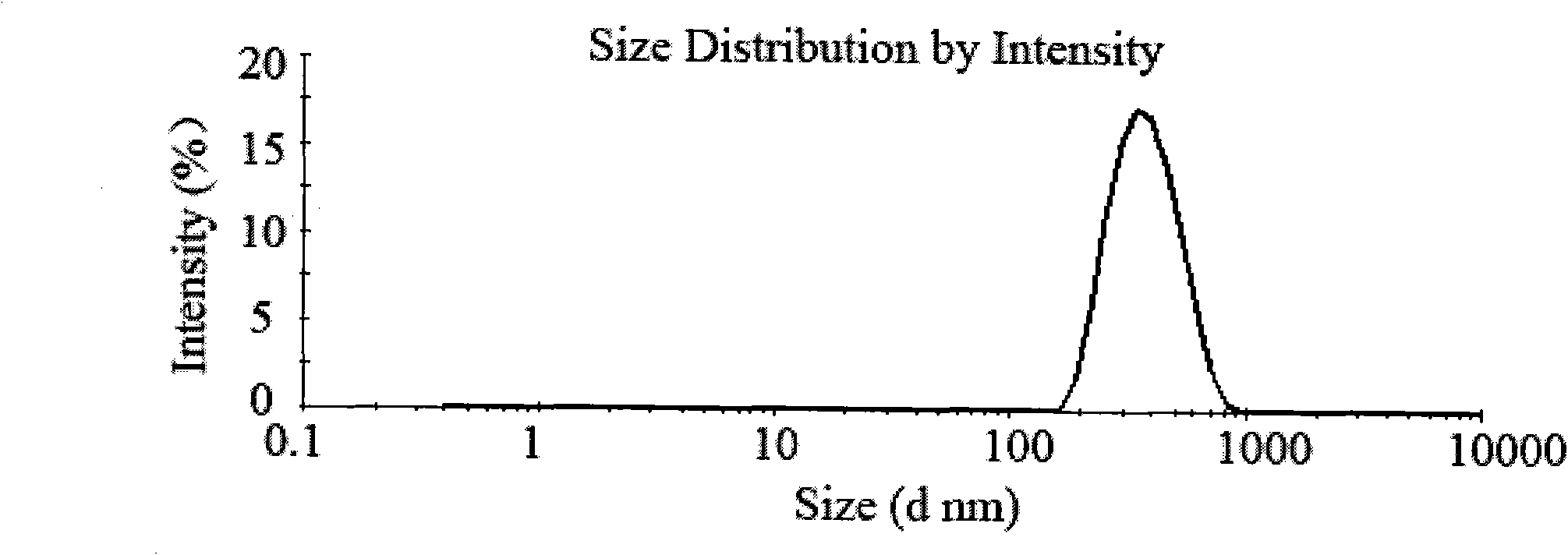
[0036] Weigh 500 mg of chitosan with a molecular weight of 100,000 Daltons and dissolve it in 50 mL of 1% acetic acid solution to obtain a 10 mg / mL chitosan solution. Prepare 20 mL of 5 mg / mL sodium polyphosphate solution with distilled water. Add acetate buffer (pH 5.5) containing 200 mg of Endostar into the chitosan solution, slowly add sodium polyphosphate solution dropwise under magnetic stirring, and react for 30 minutes to obtain recombinant human endostatin nanoparticles. The above nanoparticle solution was centrifuged at 4°C and 10,000 rpm for 30 minutes to separate the nanoparticles. The obtained nanoparticles were redispersed in 50 mL of water, 1 g of trehalose was added, and freeze-dried to obtain recombinant human endostatin nanoparticles with uniform particle size distribution.
[0037] The supernatant solution after centrifugation was appropriately diluted, and the BCA method was used (the kit was from ThermoScientific, named BCA TM Protein Assay Kit) was used...
Embodiment 2
[0039]Weigh 500 mg of chitosan with a molecular weight of 100,000 Daltons and dissolve it in 50 mL of 1% acetic acid solution to obtain a 10 mg / mL chitosan solution. Prepare 20 mL of 5 mg / mL sodium polyphosphate solution with distilled water. Take 200mg of Endostar acetate buffer solution (pH5.5) and add it to the chitosan solution, slowly add sodium polyphosphate solution dropwise under magnetic stirring, react for 5 minutes, and ultrasonicate the probe at 100W for 2 minutes to obtain recombinant human blood vessels Endostatin nanoparticles. The above nanoparticle solution was centrifuged at 4°C and 10,000 rpm for 30 minutes to separate the nanoparticles. The obtained nanoparticles were redispersed in 50 mL of water, 1 g of trehalose was added, and freeze-dried to obtain recombinant human endostatin nanoparticles with uniform particle size distribution.
[0040] The determination of drug loading is the same as in Example 1. The freeze-dried nanoparticles were redissolved i...
Embodiment 3
[0042] Weighing 250 mg of chitosan with a molecular weight of 100,000 Daltons was dissolved in 50 mL of 1% acetic acid solution to obtain a 5 mg / mL chitosan solution. Prepare 15 mL of 5 mg / mL sodium polyphosphate solution with distilled water. Add 200 mg of Endostar phosphate buffer solution (pH5.0) into the chitosan solution, slowly add sodium polyphosphate solution dropwise under propeller stirring (300 rpm), react for 10 minutes, and ultrasonically probe for 2 minutes under 100W power, that is Recombinant human endostatin nanoparticles were obtained. The nanoparticle solution was centrifuged at 4000 rpm for 10 minutes with an ultrafiltration membrane with a molecular weight cut-off of 50,000 Daltons to separate the nanoparticles. The obtained nanoparticle concentrate was dispersed in 30 mL of water, 1.5 g of trehalose was added, and freeze-dried to obtain recombinant human endostatin nanoparticles with uniform particle size distribution.
[0043] The ultrafiltrate after c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com