Method for producing human insulin growth factor-1 in recombinant Escherichia coli
A technology for recombining Escherichia coli and growth factors, applied in the field of biomedicine, can solve problems such as high cost, prevent large-scale production of hIGF-1, etc., and achieve the effects of promoting the formation of disulfide bonds, improving soluble expression, and simple separation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
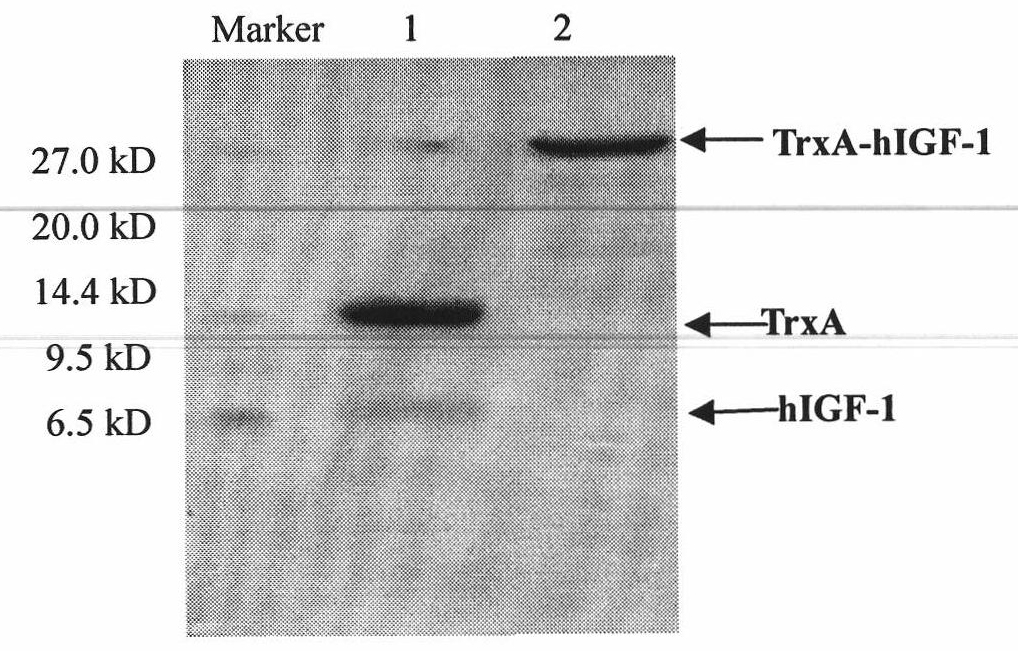
[0047] Take 5ul of the bacterial solution in the glycerol tube and add it to 5ml of LB culture solution containing ampicillin (Amp), chloramphenicol (Chl), kanamycin (Kan) and tetracycline (Tet), at 37°C, 200 rpm, Incubate overnight on a shaker. The next day, take 1ml of the bacterial liquid, add it to LB medium containing 10 times the corresponding antibiotic, and cultivate it at 37°C until OD=1.0, and the concentration of the inducer is 1.0mM. , 34°C, 37°C and 40°C for 4 hours. The optimum temperature for expression was 34°C, and the expression level of the soluble fusion protein reached 1.2g / L ( figure 2 ).
Embodiment 2
[0049] Same as in Example 1, inoculate 1ml of the bacterial solution the next day, add it to 10 times the LB medium containing the corresponding antibiotic, determine the growth curve of the engineering bacteria at 34°C, and take the early, middle, late and stable phases of the logarithmic growth phase respectively Induction was carried out, the culture temperature was 34°C during induction, the concentration of inducer was 1.0mM, and cultured for 4h after induction.
[0050] The optimum induction time was in the middle of the logarithmic growth phase, that is, after 4 hours of inoculation, the expression level of the soluble fusion protein reached 1.56g / L ( image 3 ).
Embodiment 3
[0052] Same as Example 1, inoculate 1ml of bacterial liquid the next day, add to 10 times LB medium containing corresponding antibiotics, culture at 34°C, and induce in mid-logarithmic growth phase, the concentrations of inducer IPTG are 0.1mM and 0.25mM respectively , 0.5mM, 0.75mM and 1.0mM, after adding the inducer, cultured at 34°C for 4h, the results are shown in Table 1.
[0053] The optimum concentration of inducer was 0.25mM, and the expression level of soluble fusion protein was 1.83g / L under this induction concentration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com