Method for synthesizing substituted pyrrole by cycloaddition reaction of 1, 3-diyne and primary amine
A technology of cycloaddition and amine compounds, which is applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, low yield, long reaction time, etc., and achieve the effects of high-efficiency reaction, simple reaction operation and high chemical reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
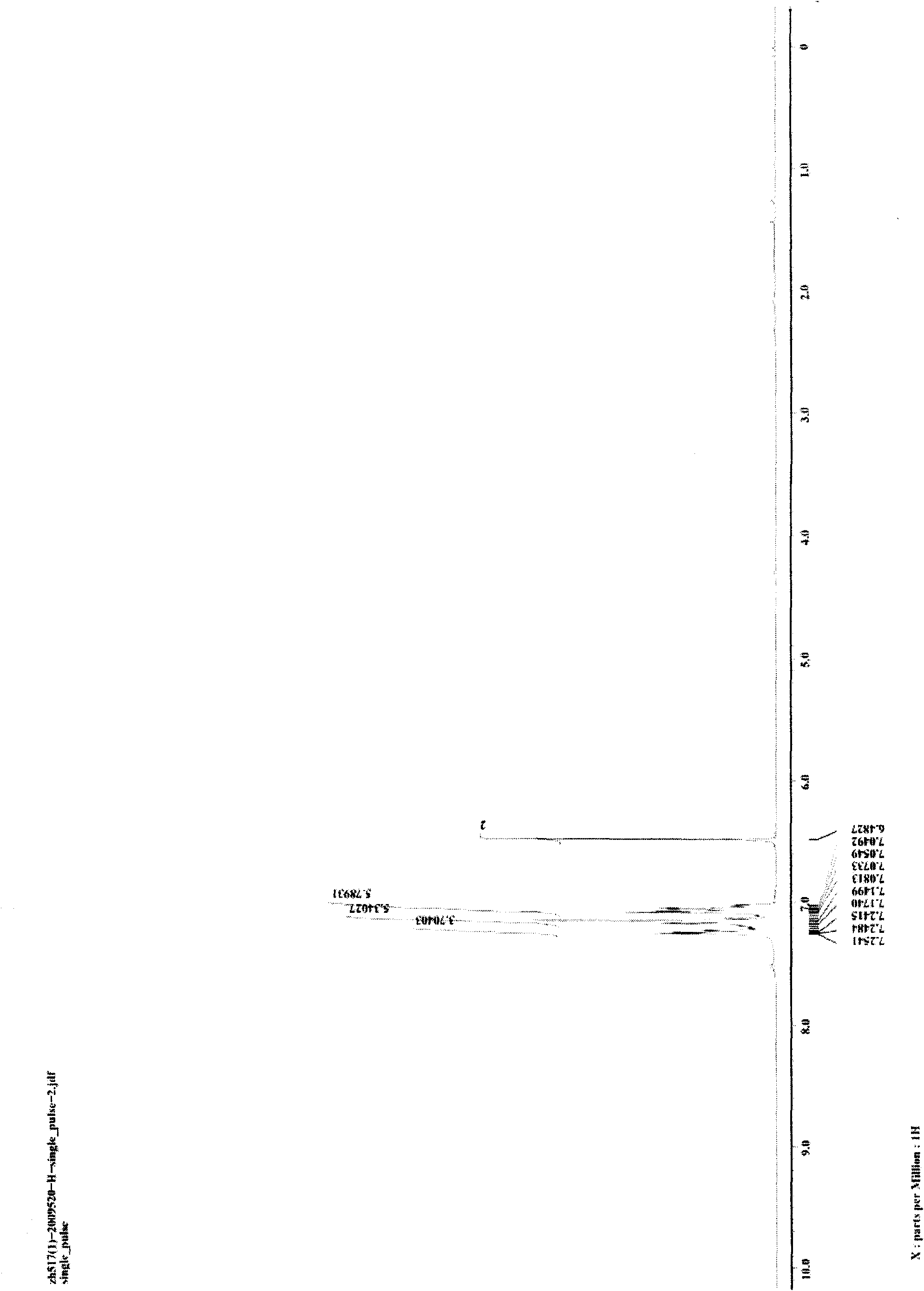
[0025] Weigh 5 mg (0.05 mmol) of CuCl and 101.0 mg (0.5 mmol) of 1,3-diphenyldiyne into a 25-mL glass reaction tube with a cover, replace the air in the reaction tube with nitrogen, and add 0.5mL of aniline, then seal the reaction tube, place the reaction tube in an oil bath and heat to 100°C, stir and keep warm for 24 hours, then cool to room temperature to obtain the target product 1,2,5-triphenylpyrrole provided by the present invention. Reaction result: the isolated target product 1,2,5-triphenylpyrrole was weighed, and the isolated yield of the product was calculated to be 96%. figure 1 and figure 2 The hydrogen spectrum and carbon spectrum of the target product 1,2,5-triphenylpyrrole prepared in this example are shown respectively. It can be seen from the figure that the structure of the compound is correct.
Embodiment 2
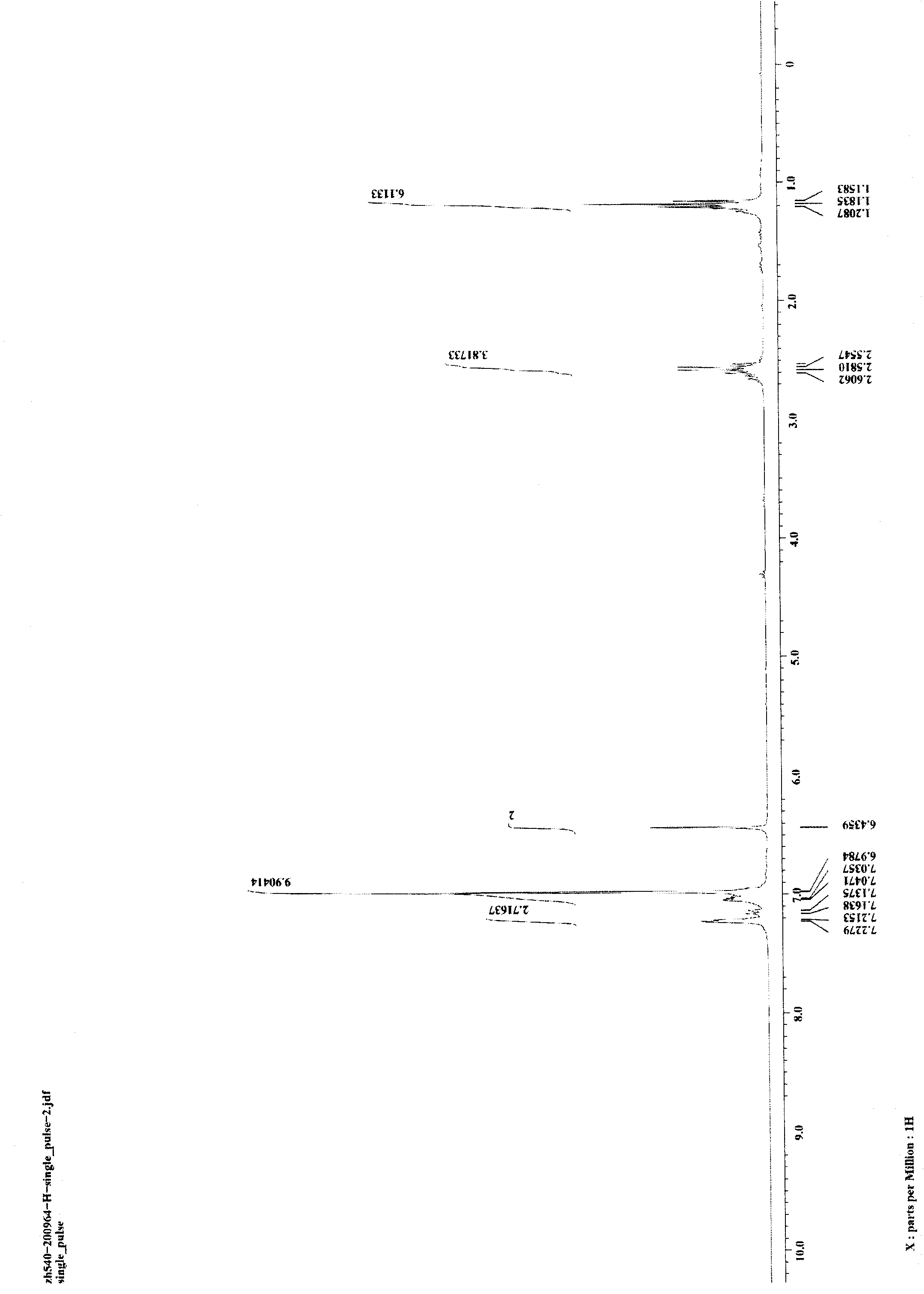
[0027] Weigh respectively 5mg (0.05mmol) of CuCl and 176.0mg (0.5mmol) of 1,3-di-p-ethylphenyldiyne into a 25-mL glass reaction tube with a cover, and replace the air in the reaction tube with nitrogen. Add 0.5mL of aniline in a nitrogen atmosphere, then seal the reaction tube, heat the reaction tube to 100°C in an oil bath, stir and keep it warm for 24 hours, and then cool to room temperature to obtain the target product 1-phenyl-2 provided by the present invention. 5-Di-p-ethylphenylpyrrole. Reaction result: the isolated target product 1-phenyl-2,5-di-p-ethylphenylpyrrole was weighed, and the isolated yield of the product was calculated to be 95%. image 3 and Figure 4 The hydrogen spectrum and carbon spectrum of the target product 1-phenyl-2,5-di-p-ethylphenylpyrrole prepared in this example are shown respectively. It can be seen from the figure that the structure of the compound is correct.
Embodiment 3
[0029] Weigh 5 mg (0.05 mmol) of CuCl and 178.0 mg (0.5 mmol) of 1,3-di-p-methoxyphenyldiyne into a 25-mL glass reaction tube with a cover, replace the air in the reaction tube with nitrogen, Add 0.5mL of aniline in a nitrogen atmosphere, then seal the reaction tube, heat the reaction tube to 100°C in an oil bath, stir and keep it warm for 24 hours, and then cool to room temperature to obtain the target product 1-phenyl-2 , 5-di-p-methoxyphenylpyrrole. Reaction result: the isolated target product 1-phenyl-2,5-di-p-methoxyphenylpyrrole was weighed, and the isolated yield of the product was calculated to be 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com