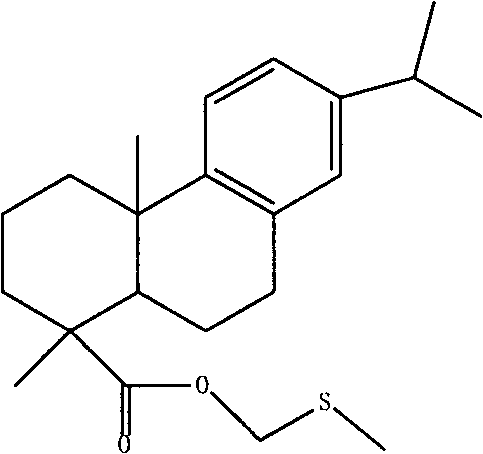
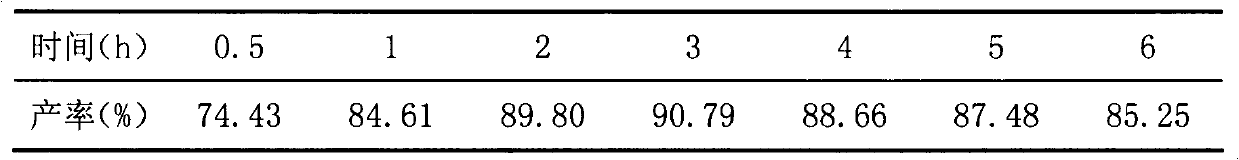
Abietic acid sulfur ether derivative and preparation method thereof
A technology of abietic acid thioether and abietic acid methylthiomethyl ester, which is applied in the field of abietic acid thioether derivatives and their preparation, can solve the problems of side reactions and high energy consumption, and achieve fast reaction speed, low acid value, and production high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Add 2g of dehydroabietic acid (6.66mmol) and 0.0080g of catalyst potassium hydroxide to a 100mL round-bottomed flask with a reflux device, then add 10.50mL (11.45g, 146.5mmol) of dimethyl sulfoxide, and react at 215°C After 3 hours, unreacted DMSO was distilled off under reduced pressure to obtain 2.5 g of a crude product with an acid value of 6.5 mg KOH / g and a yield of 104%. Add 20mL of absolute ethanol to the crude product, stir and reflux in a magnetic heating stirrer to dissolve, add concentrated potassium hydroxide solution dropwise while stirring, adjust the pH of the solution to 9-10, and then reflux for half an hour to make potassium hydroxide and dehydrogenated arbores The acid is fully reacted, after cooling to room temperature, add distilled water to wash thoroughly, then filter with suction, add the filter cake to 0.03mol / L potassium hydroxide solution and stir at 50°C for 1-2 hours, filter with suction and wash with distilled water several times , to obtai...
Embodiment 2
[0062] Put 2g of common rosin (6.61mmol) and 0.0080g of potassium hydroxide in a 100mL round-bottomed flask with a reflux device, then add 10.50mL (11.45g, 146.5mmol) of dimethyl sulfoxide, and react at 215°C for 3 hours. Unreacted DMSO was distilled off under reduced pressure. The obtained crude product was 2.23g, and the yield was 92.9%. The acid value of the product was 6.39 mg KOH / g. The crude product was purified according to the method of Example 1 to obtain 1.42 g of the product with a yield of 59.17% and an acid value of 2.40 mg KOH / g.
Embodiment 3
[0064] Put 2g of disproportionated rosin and 0.0080g of potassium hydroxide in a 100mL round-bottomed flask with a reflux device, then add 10.50mL (11.45g, 146.5mmol) of dimethyl sulfoxide, react at 215°C for 3 hours, and distill under reduced pressure. Remove unreacted DMSO. The obtained crude product was 2.28g, and the yield was 95.0%. The acid value of the product was 5.56 mg KOH / g. The crude product was purified according to the method of Example 1 to obtain 1.46 g of the product with a yield of 60.83% and an acid value of 2.38 mg KOH / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com