T vector for screening solubility expression of protein and construction method and application thereof
A soluble and carrier technology, applied in the field of genetic engineering, can solve the problems of low fluorescence contrast, fluorescence retardation, low fluorescence display intensity, etc., and achieve the effect of convenient screening.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
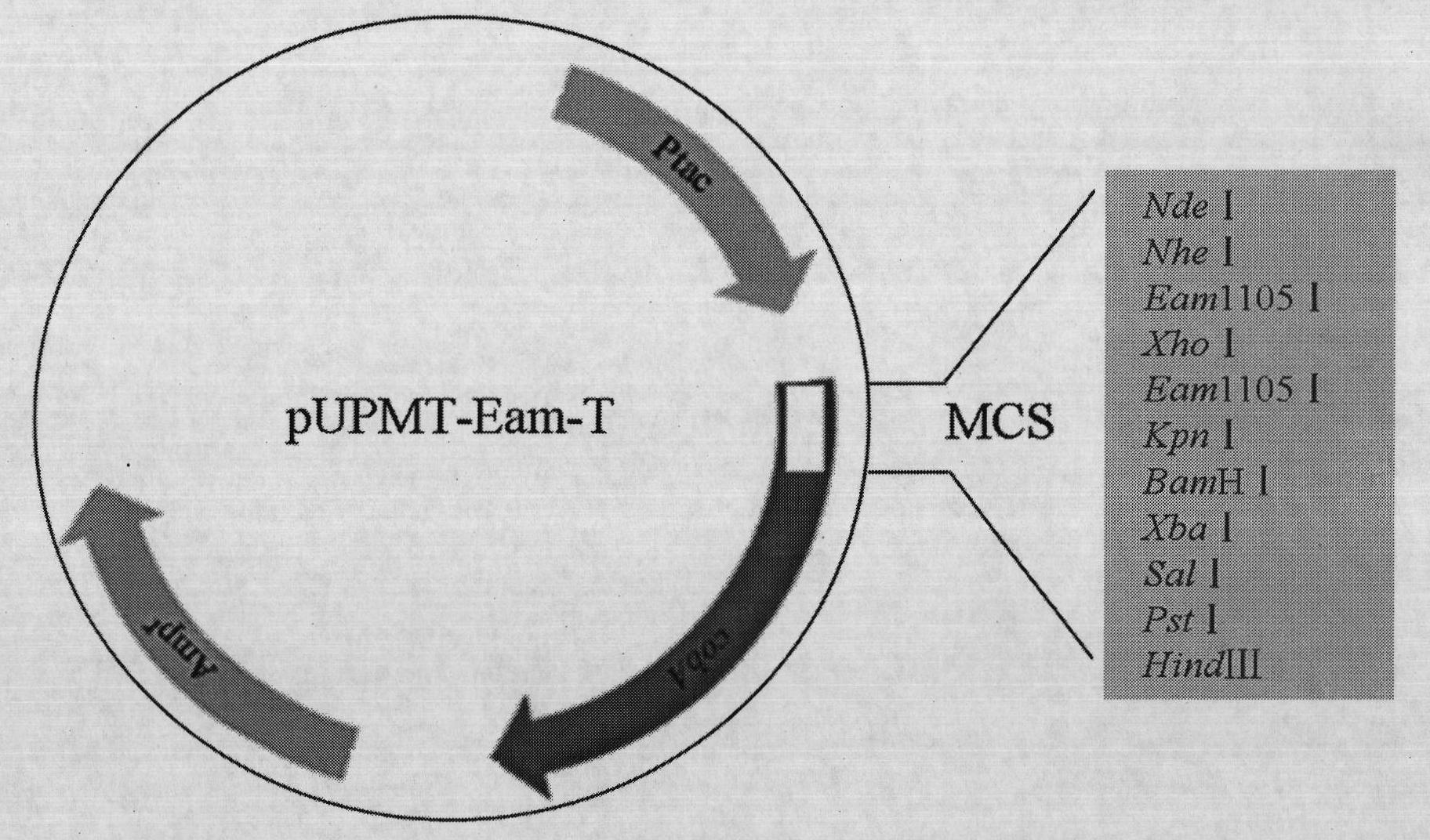
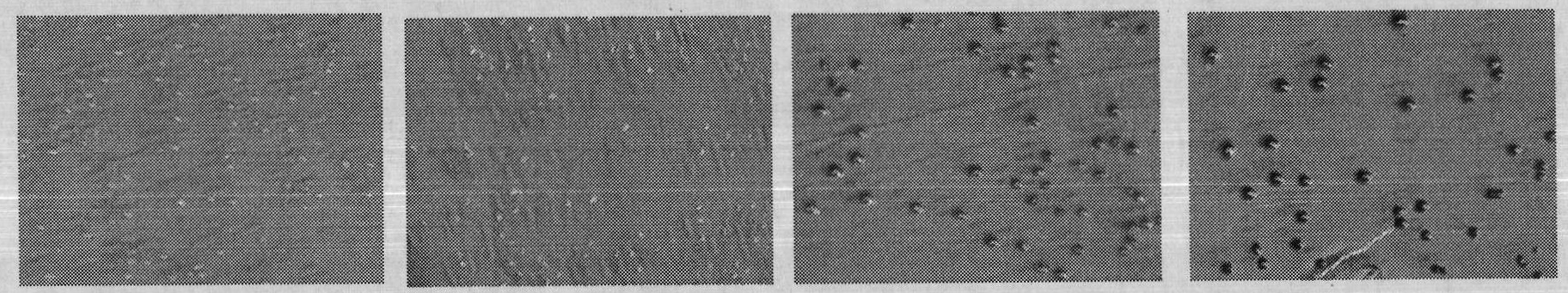
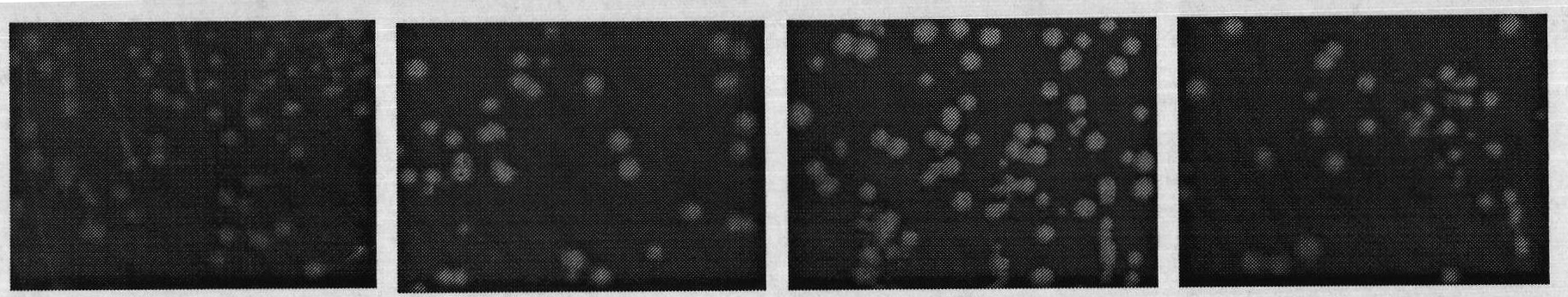
[0032] 1. Verification of the correlation between C-terminal fusion α-peptide and protein water solubility
[0033] (1) Three mutants of maltose-binding protein (MBP) were constructed according to literature reports: folding-deficient type and two inclusion body MBP mutants (Betton JM, Hofnung M. Folding of a mutant maltose-binding protein of Escherichia coli which forms inclusion bodies. The Journal of Biological Chemistry.1996,271(14):8046-8052; Bajaj K, Chakrabarti P, VaradarajanR. Mutagenesis-based definitions and probes of residue burialin proteins.Proc Natl Acad Sci USA.2005,102(45):16221 -16226.).
[0034] (2) Construction of fusion expression vectors of C-terminal and α-peptide of wild-type and mutant MBP. The fusion expression vector of wild-type MBP and α-peptide is named MBP WT; the fusion expression vector of folding-deficient MBP and α-peptide is named G32D, and the glycine (G) at position 32 of maltose-binding protein (MBP) is mutated to natural Aspartic acid (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com